First Report of Gray Mold Disease of Sponge Gourd (Luffa cylindrica) Caused by Botrytis cinerea in Korea
Article information
Abstract
In October 2014, an occurrence of gray mold was observed on young fruits of sponge gourd (Luffa cylindrica) in Sachunmun, Gangneung, South Korea. Symptoms included abundant mycelia growth with gray conidia on young fruits and finally rotting the fruits. The fungus was isolated from symptomatic fruits and its pathogenicity was confirmed. Based on the morphological features and sequence analysis of ITS-5.8S rDNA, G3PDH, HSP60, and RPB2 genes, the pathogen was identified as Botrytis cinerea Pers. This is the first report of gray mold caused by B. cinerea on L. cylindrica in Korea.
Introduction
Sponge gourd (Luffa cylindrica L.) is a tropical and subtropical vegetable belongs to the family Cucurbitaceae. Luffa gourds are grown primarily for their fibrous tissue skeleton, which is commonly used as scrubbers, cleaning pads or bath sponges, but green immature fruits were cooked as vegetable and eaten as squash or substituted for cucumber in salads (Oboh and Aluyor, 2009). Botrytis cinerea (teleomorph Botryotinia fuckeliana) is an air borne, ubiquitous filamentous fungal pathogen and may cause gray mold on a variety of dicotyledonous plants, including many vegetables, fruits, ornamental flowers, and greenhouse plants (Elad, 1997; Jarvis, 1977). The pathogen is a necrotroph, inducing host cell death resulting in serious damage to plant tissues culminating in rot of the plant (Govrin and Levine, 2000; Staats et al., 2005). Botrytis infections are favored by a cool weather, rainy spring season and summer temperatures of approximately 15°C (60°F). Gray mold can be particularly damaging when rainy and/or damp weather occurs over several days. Since 2014, gray mold of sponge gourd caused by B. cinerea has been found at different locations in Korea. The aim of the present study was to identify the causal agent associated with gray mold observed on sponge gourd in Korea, based on culture characteristics, molecular phylogenetics, and pathogenicity.
Isolation of the fungi and pathogenicity test
In October 2014, several young fruits exhibiting symptoms with gray mold were observed in Sachunmun, Gangneung, Gangwon Province, Korea. Symptoms included abundant mycelia growth with gray conidia on young fruits and finally rotting the fruits (Fig. 1A). Diseased fruit tissue was excised and surface sterilized by immersion in 0.1% sodium hypochlorite (NaOCl) for 1 minute, rinsed three times with sterilized distilled water, placed on potato dextrose agar (PDA; Difco, Detroit, MI, USA) plates, and incubated at 20°C±2°C.
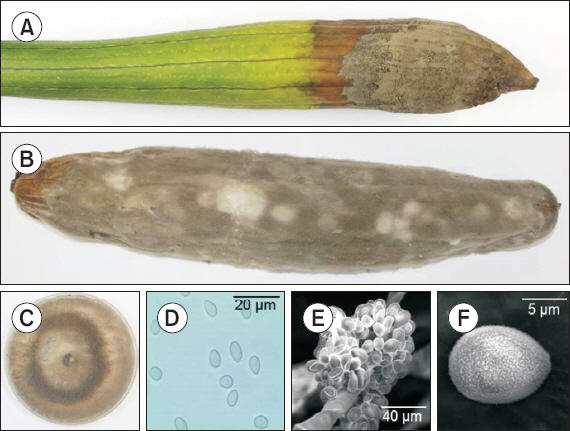
Gray mold caused by Botrytis cinerea on sponge gourd (Luffa cylindrica). (A) Young fruit damaged by gray mold. (B) Gray mold developed 7 days after artificial inoculation. (C) Two-week-old colony of B. cinerea on potato on potato dextrose agar. (D) Conidia. (E, F) Conidiophore and conidia of B. cinerea (scanning electron microscopy).
To conduct the pathogenicity test, inoculum was prepared by harvesting conidia from 2-week-old cultures on PDA. A conidial suspension (2×106 conidia/ml) was sprayed onto 3 young fruits that were wounded by piercing with a sterilized needle. Another 3 wounded fruits were sprayed with sterilized water, serving as controls. Inoculated all fruits were sealed in a plastic bags that had been sprayed with water on the inside to maintain high humidity and incubated in growth chamber at 20°C±2°C. After 7 days, blackish gray mycelia with abundance conidia were developed on inoculated fruits (Fig. 1B); whereas control fruits remained symptomless. The pathogenicity test was carried out twice with similar results. The pathogen was successfully re-isolated from inoculated fruits, fulfilling Koch's postulates. The results showed that B. cinerea was the causal agent of the disease.
DNA extraction, polymerase chain reaction (PCR) amplification, and sequence analysis
Genomic DNA of isolated fungus was extracted using DNeasy Plant Mini Kit (Qiagen Inc., Valencia, CA, USA) following the manufacturer's instructions. DNA sequence of the internal transcribed spacers (ITS) and 5.8S ribosomal DNA (ITS-5.8S rDNA) ITS4 (White et al., 1990) and three nuclear protein-coding genes: glyceraldehyde-3-phosphate dehydrogenase gene (G3PDH), heat-shock protein 60 gene (hsp60) and DNA-dependent RNA polymerase subunit II gene (RPB2) were amplified using primer pairs ITS1 (5′-TCCGTAGGTGAACCTGCGG- 3′)/ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al., 1990), G3PDH-F (5′-ATTGACATCGTCGCTGTCAACGA-3′)/G3PDH-R (5′-ACCCCACTCGTTGT CGTACCA-3′), HSP60-F (5′-CAACAATTGAGATTTGCCCACAAG-3′)/HSP60-R (5′-GATGGATCCAGTGGTACCGAGCAT-3′), and RPB2-F (5′-GATGATCGTGATCATTTCGG-3′)/RPB2-R (5′-CCCATAGCTTGCTTACCCAT- 3′) (Staats et al., 2005), respectively. The PCR was performed in a 25 μl reaction mixture containing 0.5 μl of each primer, 0.5 μl of TaqDNA polymerase (Bioneer, Daejeon, Korea), 0.5 μl of each dNTP, 2.5 μl of 10× PCR reaction buffer, 18.5 μl of distilled water, and 2.0 μl of template DNA. The reaction was performed in Mastercycler Gradient (Eppendorf, Hamburg, Germany). The following thermo cycling pattern was used to amplify ITS region: an initial preheat at 94°C for 4 minutes, followed by 35 cycles of denaturation at 94°C for 35 seconds, annealing at 52°C for 55 seconds, and extension at 72°C for 1 minute, terminating with a final extension at 72°C for 10 minutes. The PCR cycle conditions were 94°C for 5 minutes, followed by 35 cycles of 94°C for 30 seconds, 55°C for 30 seconds, 72°C for 90 seconds and a final elongation step at 72°C for 10 minutes for HSP60 and RPB2 gene segments. The same program with an annealing temperature of 64°C was applied to G3PDH gene fragments. The obtained nucleotide sequences were searched by using BLASTn available from the GenBank database (http://www.ncbi.nlm.nih.gov/BLAST/). Phylogenetic analysis was performed by using the MEGA5 program (Tamura et al., 2011) with the neighbor-joining method (Saitou and Nei, 1987).
Identification and characterization of B. cinerea
A total of 4 morphologically similar isolates were obtained from 2 diseased tissue samples and isolate SGGM003 was examined for identification. Fungal colonies on PDA at 20°C were initially white and turned grey to dark grey after 5 days (Fig. 1C). Conidia (n=50) were single-celled, ellipsoidal or ovoid, 5.8–7.9×5.8–9.5 μm on naturally infected fruits and 5.8–10.2×4.8–7.2 μm on PDA (Fig. 1D). Conidiophores arose singly or in groups, straight or flexuous, septate, with an inflated basal cell and brown to light brown, and measured 11.2–25.5×112.3–415.7 μm. After three weeks, the fungus formed several black sclerotia ranging from 1.0–4.3×1.2–3.5 mm (n=20) near the edge of the Petri dish. The morphological characteristics of the identified species are summarized in Table 1.
BLAST analysis of the resulting approximately 527-bp ITS-5.8S rDNA sequence, 883-bp G3PDH sequence, 976-bp HSP60 sequence and 1,093-bp RPB2 sequence were obtained. The ITS-5.8S rDNA, G3PDH, HSP60 and RPB2 nucleotide sequences for the re-isolated fungus were also obtained, and were 100% identical to the sequences of original isolates. The sequences for the representative isolate SGGM003 have been deposited in the NCBI database (GenBank accession Nos. KT728905 for ITS-5.8S rDNA, KT728906 for G3PDH, KT728907 for HSP60, and KT728908 for RPB2). Blast analysis confirmed the identity of the fungus, showing 100% similarity to the ITS-5.8S rDNA sequence of published B. cinerea isolate Bau42_3 (GenBank accession No. KP903573.1), 100% identity to the G3PDH sequence of isolate GRGM-3 of B. cinerea (KT025631.1), 100% identity to the HSP60 sequence of isolate SD1 of B. cinerea (KM016534.1), and 100% identity to the RPB2 sequence of isolate B326 of B. cinerea (KF857477.1). In the phylogenetic tree based on combined G3PDH, HSP60, RPB2 nucleotide sequences, the representative isolate was placed within a clade comprising reference isolates of B. cinerea (Fig. 2).
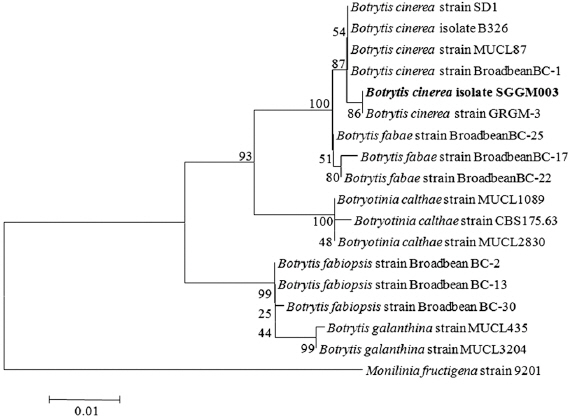
Phylogenetic analysis of Botrytis cinerea isolate SGGM003, constructed by using the neighbor-joining method based on combined G3PDH, HSP60, and RPB2 gene sequence data. Monilinia fructigena was used as the out group. The numbers at the nodes indicate bootstrap values from a test of 1,000 replicates. The scale bar indicates the number of nucleotide substitutions.
Based on symptoms, mycological characteristics, molecular data and pathogenicity, this fungus was identified as B. cinerea Pers. (Barnett and Hunter, 1972; Ellis and Waller, 1974; Zhang, 2006). Gray mold disease of L. cylindrica caused by B. cinerea has been recorded in Taiwan (Ko et al., 2007). To our knowledge, this is the first occurrence of gray mold on sponge gourd in Korea.
