First Report of Pectobacterium aroidearum Causing Soft Rot on Ficus carica in Korea
Article information
Abstract
In July 2021, symptoms of soft rot were observed on the stems of Ficus carica in Yeongam, Jeollanamdo, Korea. To accurately diagnose the cause, infected stem was collected and bacterial strain was isolated. Among these, the pathogenic strain KNUB-08-21 was identified as Pectobacterium aroidearum through 16S rRNA gene sequencing and phylogenetic analysis based on the concatenated sequences of the dnaX, leuS, and recA genes. The affiliation of the isolate with this bacterial species was also confirmed by its biochemical characteristics obtained using API ID 32 GN system. Artificial inoculation confirmed the strain's pathogenicity in figs, causing significant damage to both stems and fruits. To our knowledge, this is the first report of P. aroidearum causing soft rot disease in F. carica in Korea.
Ficus carica, also known as the fig, is a member of the Moraceae family, which includes around 40 genera. The Ficus genus is one of the largest angiosperm genera, comprising over 800 species of trees, epiphytes, and shrubs found in tropical and subtropical regions globally (Singh et al., 2011). The Asian-Australasian region is home to the highest diversity, with approximately 500 Ficus species (Badgujar et al., 2014). In 2020, Korea produced about 3,460 tons of figs (Statistics Korea, 2023). Only a few bacterial diseases have been reported in Ficus species, particularly edible figs. These diseases include crown gall caused by Agrobacterium tumefaciens; Pseudomonas leaf spot caused by Pseudomonas cichorii; and Xanthomonas leaf spot caused by Xanthomonas campestris pv. fici (Bouzar and Jones, 2001; Campoverde and Palmateer, 2011; Elboutahiri et al., 2009). Additionally, Agrobacterium larrymoorei was isolated from gall formations on weeping figs (Mousavi et al., 2020). However, while soft rot is a disease reported worldwide, there has been limited research on Ficus species. Bacterial soft rot is a common disease in agricultural ecosystems (Charkowski, 2018). It primarily affects plant storage organs like tubers, rhizomes, and bulbs (Ma et al., 2007), but can also appear in fleshy plant organs such as succulent stems and leaves or densely packed leaf vegetables like lettuce (Ma et al., 2007). The pectinolytic soft rot Pectobacteriaceae, a group of bacterial plant pathogens, consists of two genera: Pectobacterium and Dickeya (Adeolu et al., 2016). Recently, Pectobacterium aroidearum has been reported to affect various plants, including alocasia, konjac, Chinese cabbage, and pumpkin (Chen et al., 2020; Mikicińsk et al., 2023; Moraes et al., 2017; Wei et al., 2020; Xie et al., 2018; Xu et al., 2020). However, P. aroidearum has never been reported to cause soft rot disease in F. carica.
In July 2021, soft rot symptoms were observed on stems of F. carica in Yeongam, Jeollanamdo, Korea (Fig. 1A, B). Diseased stems were collected for isolation of pathogens to an accurate diagnosis. Infected tissue fragments were immersed in a 5 ml solution of saline (0.8% NaCl) for 20 min. The resulting suspension was then divided into 50 µl and spread onto nutrient agar (NA; Difco, Detroit, MI, USA) media, followed by an incubation period of 48–72 hr at 30°C. After 3 days, white-gray circular colonies exhibiting the typical cultural characteristics of bacterial strains were obtained on NA. Single colonies were picked, purified by repeated streaking on fresh NA plates, and a randomly chosen strain, designated as KNUB-08-21, was used for further comprehensive analysis.

Soft rot symptoms on Ficus carica stems. (A, B) Soft rot caused by Pectobacterium aroidearum KNUB-08-21 on F. carica in the field in Yeongam, Jeollanamdo, Korea. (C) Soft rot induced by P. aroidearum KNUB-08-21 through artificial inoculation on the stems. (D) Sterilized water was used as a control.
To test the pathogenicity of the bacterial strain, surface-sterilized stems with holes in the center were filled with 100 µl of bacterial suspension (1×109 cells/ml) of strain KNUB-08-21 to test the ability to cause soft rot. As a control, a mock infection was conducted by inoculating the stem with 100 µl of distilled water. Inoculated plants were kept at 25°C and 80% relative humidity. Three days later, bacterial strain caused symptoms similar to those in the field (Fig. 1C). In contrast, the control did not exhibit any noticeable symptoms (Fig. 1D). Based on the result of the pathogenicity test, the bacterial strain, designed KNUB-08-21, was selected for further detailed investigation.
To identify strain KNUB-08-21, genomic DNA was extracted from it utilizing the HiGene Genomic DNA Prep Kit (Biofact, Daejeon, Korea) in accordance with the manufacturer's instructions. Subsequently, the 16S rRNA gene was subjected to polymerase chain reaction (PCR) employing the 9F/1512R primers as outlined by Weisburg et al. (1991). The 16S rRNA gene of strain KNUB-08-21 was sequenced and found to be comprised of 1,351 base pairs in total length (GenBank accession no. LC779908). A BLAST search in the NCBI data-base showed a high similarity between the 16S rRNA gene sequence of KNUB-08-21 and those of P. aroidearum CEP2 (GenBank no. MN904952) (99.56%), P. carotovorum HG-49 (GenBank no. CP032619) (99.56%), and P. colocasium PL155 (GenBank no. CP118921) (99.19%). This result suggests that strain KNUB-08-21 belongs to the genus Pectobacterium. However, an accurate identification of the isolate solely based on 16S rRNA gene was not achievable.
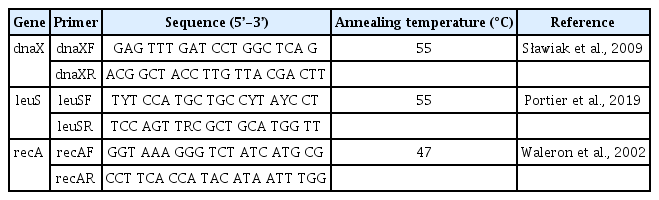
For precise identification of the isolated Pectobacterium strain, three housekeeping genes (dnaX, leuS, and recA) were amplified using the protocols and primers previously reported by Portier et al. (2020). The strain KNUB-08-21 was amplified using dnaXF/ dnaXR, leuSF/ leuSR, and recAF/ recAR primers to analyze dnaX, leuS, and recA genes (Table 1). Multiple sequence alignment (dnaX, 480 bp; leuS, 530 bp; recA, 609 bp) was executed utilizing the MEGA7 software program (Kumar et al., 2016). The GenBank accession numbers of reference sequences of Pectobacterium species used in this study are shown in Table 2. A well-supported monophyletic clade, consisting of strain KNUB-08-21 and several members of P. aroidearum (CFBP 1457, CFBP 2573, CFBP 6725, and CFBP 8737), strongly indicates their belonging to the same species (Fig. 2).
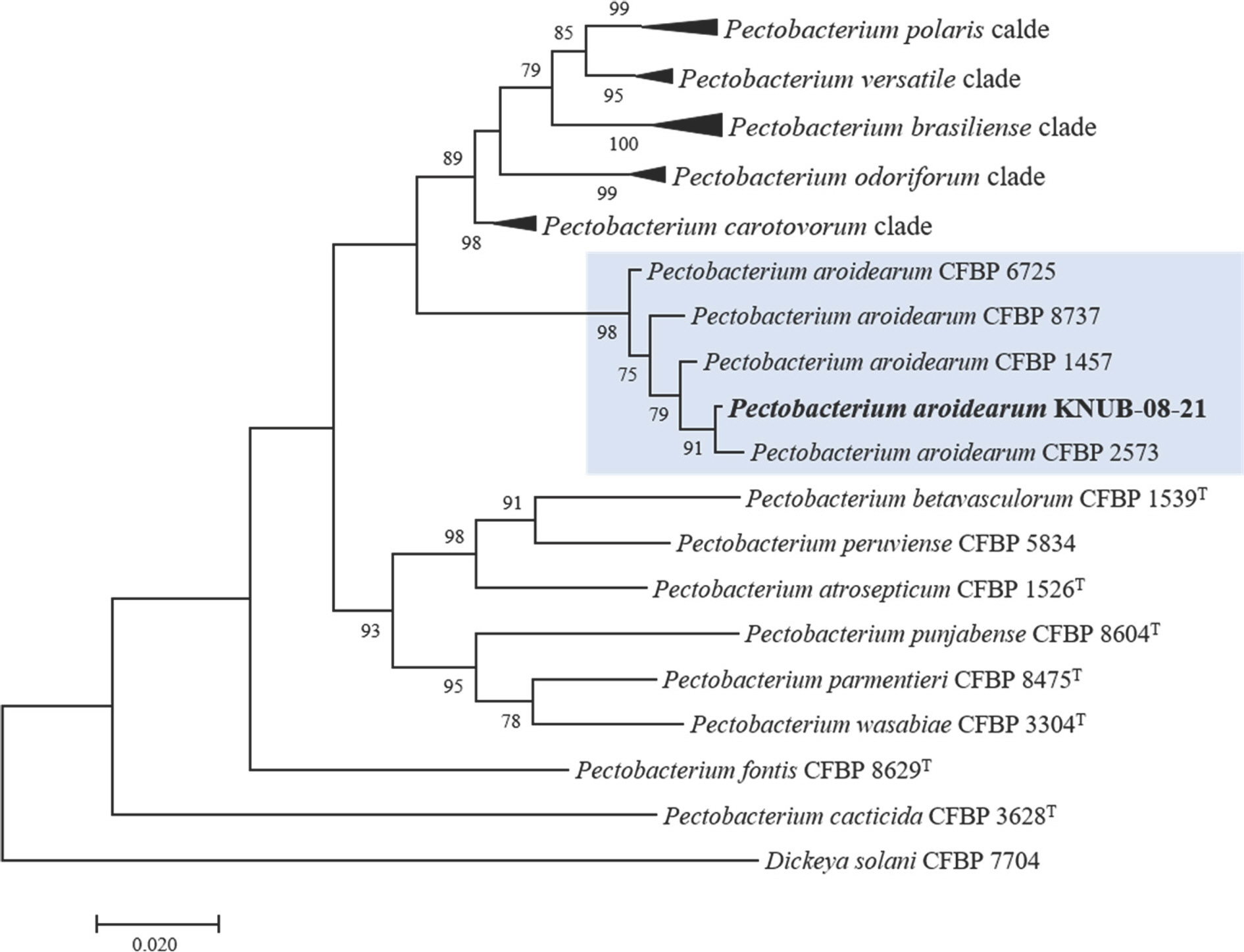
Maximum-likelihood phylogenetic tree showing the relationship between Pectobacterium aroidearum KNUB-08-21 and other Pectobacterium species based on concatenated sequences of dnaX, leuS, and recA genes. Bootstrap values (based on 1,000 replications) more than 70% are displayed on the branch points. Dickeya solani CFBP 7704 was used as the outgroup. Scale bar: 0.020 substitutions per nucle-otide position.
The isolate KNUB-08-21 underwent compound utilization analysis utilizing the API ID 32 GN system (Biomérieux, Marcy l'Etoile, France) in accordance with the manufacturer's instructions. The results showed that strain KNUB-08-21 was able to utilize N-acetyl-glucosamine, L-arabinose, D-glucose, inositol, D-mannitol, D-melibiose, L-rhamnose, L-serine, and sucrose. However, it was unable to utilize L-alanine, glycogen, L-histidine, itaconic acid, lactic acid, D-maltose, L-proline, propionic acid, and valeric acid. The strain KNUB-08-21 displayed almost all the characteristic features consistent with the type strain of P. aroidearum described by Nabhan et al. (2013), except for its inability to metabolize D-glucose and lactic acid (Table 3). This difference can be considered as intraspecific variability among various strains belonging to P. aroidearum. Overall, the outcomes of conventional biochemical tests corroborate the molecular analysis results, thus confirming the accurate identification of strain KNUB-08-21 as P. aroidearum.
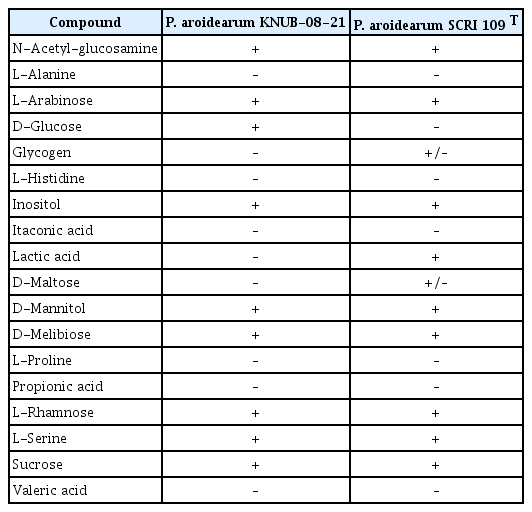
Utilization of various compounds as the sole carbon source by Pectobacterium aroidearum KNUB-08-21 and Pectobacterium aroidearum SCRI 109 T
To investigate whether the pathogenicity of the P. aroidearum KNUB-08-21, as confirmed in F. carica, can induce disease symptoms in other parts of the plant besides the stems. Artificial inoculation was conducted on the fruit to determine the potential development of symptoms. Before inoculation, the fruit surfaces were sterilized using 70% ethanol and subsequently rinsed with distilled water. Inoculation was carried out using a 100 µl suspension of P. aroidearum KNUB-08-21 at a concentration of 1×108 cells/ml. As a control, a mock infection was conducted by inoculating the fruit with 100 µl of distilled water. The inoculated fruits were stored at 25°C with humidity levels exceeding 80%. After 2 days, symptoms of soft rot began to appear in the fruits inoculated with the suspension of P. aroidearum KNUB-08-21, accompanied by the onset of a foul odor. After 5 days, the fruits began to split, revealing soft and decayed internal tissues (Fig. 3A). After 7 days, the fruits completely detached from the stems and fell to the ground. Furthermore, the interior of the infected fruits displayed typical symptoms of soft rot (Fig 3B). In contrast, control plants showed no infectious symptoms (Fig. 3C, D).

Pathogenicity of Pectobacterium aroidearum KNUB-08-21 on fruits of Ficus carica. (A) Fruits inoculated with P. aroidearum KNUB-08-21 suspension exhibit onset of soft rot symptoms, accompanied by a putrid smell. Upon splitting open, internally soft and decayed tissues be-come visible. (B) Characteristic symptoms of soft rot are evident within infected fruits. (C, D) Control plants display no infection symptoms.
Several strains belonging to P. aroidearum have primarily been isolated from monocotyledonous plants, including Zantedeschia aethiopica in South Africa, Saccharum spp. in Jamaica, Persea americana, Ornithogalum dubium in Israel, and Amorphophallus konjac in East Asia (Li et al., 2022; Nabhan et al., 2013; Sun et al., 2019). Recent studies have confirmed the presence of P. aroidearum in several plant species, including alocasia, konjac, Chinese cabbage, and pumpkin (Chen et al., 2020; Mikicińsk et al.,2023; Moraes et al., 2017; Wei et al., 2020; Xie et al., 2018; Xu et al., 2020). However, there have been no recorded cases of P. aroidearum causing soft rot disease in figs until now.
Through comprehensive analysis encompassing 16S rRNA gene sequence analysis, multilocus sequence analysis, and meticulous assessment of physiological characteristics, P. aroidearum was confirmed as the causative agent isolated from F. carica, which represents soft rot disease in Korea. Moreover, it was noted that symptomatology presents as stem browning; however, when inoculated within the fruit, the disease swiftly advances over time, resulting in substantial damage. Additionally, substantiation has been made that inoculating at distinct anatomical sites within the host can yield divergent levels of disease severity. These findings corroborate the results of prior research demonstrating that the genus Pectobacterium can induce soft rot in various anatomical regions (Ma et al., 2007).
Our findings enhance the understanding of the diversity of P. aroidearum linked with F. carica and underscore the importance of timely detection and strategic management protocols to impede the spread of the pathogen. Further investigations are imperative to scrutinize the epidemiology and ecology of P. aroidearum in areas dedicated to F. carica production. Moreover, there is a pressing need to devise efficacious control measures aimed at mitigating the economic repercussions induced by this pathogenic agent. This is pivotal for diminishing potential losses in the agricultural sector.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. RS-2021-RD010123)” Rural Development Administration, Republic of Korea.