First Report of Pectobacterium aroidearum Causing Soft Rot on Zamioculcas zamiifolia
Article information
Abstract
Zamioculcas zamiifolia is a popular indoor ornamental plant in Korea. In August 2021, a severe outbreak of soft rot disease affected Z. zamiifolia in Emseong, Chungcheongbuk-do, Korea. Infected plants displayed wilting, water-soaked lesions, stem collapse, and green-brown discoloration. The bacterial strain KNUB-05-21 was isolated from infected stems and identified as Pectobacterium aroidearum using 16S rRNA nucleotide sequencing and multilocus sequence analysis based on partial sequences of dnaX, leuS, and recA genes. Confirmation of its affiliation with P. aroidearum was also obtained through biochemical and morphological characterization. To confirm the pathogenicity of strain KNUB-05-21, its suspension was injected into Z. zamiifolia stems. Within a week, soft rot developed on the stems, exhibiting symptoms similar to those observed in field-infected plants. The reisolated strain was identical to those of P. aroidearum. Before this study, P. aroidearum was not reported as a causative pathogen of Z. zamiifolia soft rot in Korea.
Zamioculcas zamiifolia, also known as ZZ plant, is a member of the Araceae family, originating from Africa and span-ning from Kenya to South Africa. This visually pleasing indoor plant is widely cultivated in Korea for its rich dark-green foli-age and remarkable adaptability to low-light conditions. In addition to its aesthetic value, Z. zamiifolia is more effective than Sansevieria spp. in purifying indoor air by eliminating pollutants such as benzene, ethylbenzene, toluene, and xylene (Toabaita et al., 2016). Despite its popularity, various diseases of Z. zamiifolia have been reported worldwide, including soft rot and foliar blight (Sanahuja et al., 2016; Seo et al., 2019; Stutz et al., 2020). Studies from Korea documented symptoms in Z. zamiifolia infected with Athelia rolfsii, including water-soaked stems, lower leaf chlorosis followed by stem collapse, and crown rot (Seo et al., 2019).
Members of the genus Pectobacterium (formerly classified as Erwinia) cause soft rot disease in plants across at least 16 families of dicotyledonous plants and 11 families of monocotyledonous angiosperms (Ma et al., 2007). Pectobacterium atrosepticum and Pectobacterium carotovorum have gar-nered significant attention due to their economic impact on potato cultivation, a crucial food source (Pérombelon, 2002). P. carotovorum exhibited substantial phenotypic, genetic, and pathogenic diversity with its subspecies, notably P. carotovorum subsp. carotovorum, causing diseases in various hosts, including carrots, cabbage, sunflower, and potatoes (Yap et al., 2004). In France, the strains responsible for witloof chicory disease were classified under P. carotovorum subsp. odoriferum (Nabhan et al., 2012a), now known as Pectobacterium odoriferum (Portier et al., 2019). A distinct subgroup contributing to potato blackleg disease, resembling symptoms induced by P. atrosepticum, was identified as P. carotovorum subsp. brasiliense (Duarte et al., 2004; Nabhan et al., 2012a), currently classified as Pectobacterium brasiliense (Portier et al., 2019). Nabhan et al. (2012b) demonstrated that five strains corresponding to the novel species (SCRI 109 T, SCRI 121, SCRI 3, SCRI 102, and Pc1) isolated from various plants (Zantedeschia aethiopica, Saccharum spp., Solanum tuberosum, Persea americana, and Ornithogalum dubium) clustered closely together with a significant bootstrap value, distinct from representatives of P. carotovorum clusters. Consequently, each SCRI 109 T, SCRI 121, SCRI 3, SCRI 102 and Pc1 strain was identified as belonging to a novel species designated as Pectobacterium aroidearum (Nabhan et al., 2013). Previous studies have reported that P. aroidearum can cause bacterial soft rot in various plant species, including Alocasia amazonica, Amorphophallus konjac, Brassica rapa, Capsicum annuum, Cucurbita pepo, Daucus carota, and Syngonium podophyllum (Mikiciński et al., 2023; Moraes et al., 2017, 2020; Tang et al., 2021; Wei et al., 2020; Xie et al., 2018; Xu et al., 2020). Symptoms displayed by plants infected with P. aroidearum have been extensively documented, with a wide range of variations. In D. carota infected with P. aroidearum, intact peels were observed above the affected rotten tissues (Tang et al., 2021). Furthermore, P. aroidearum infection in A. amazonica manifested as beige, necrotic, hydrated spots that spread to the leaf blades, causing maceration and plant death (Mikiciński et al., 2023). P. aroidearum infection in B. rapa resulted in soft rot symptoms, including dark-brown lesions on the stems, brown water-soaked appearance, oozing of creamy white substance in severely infected plants, and yellow-brown symptoms in the leaves, eventually leading to rot (Teoh et al., 2023). However, until this study, P. aroidearum was not documented as the causative agent of bacterial soft rot in Z. zamiifolia.
In August 2021, Z. zamiifolia samples exhibiting a wilted appearance, water-soaked lesions, and stem collapse were found in two nurseries in Emseong, Chungcheongbuk-do, Korea (Fig. 1A). In Fig. 1B, infected stems turned green– brown. To isolate the pathogens, the interfaces between infected and healthy tissues were treated with 70% ethyl alcohol for surface sterilization, followed by thorough rinsing with sterile distilled water. Bacterial cells were allowed to move into the solution by soaking each tissue in 2 ml sterile distilled water for 2 min. A 100 μl liquid suspension was spread onto the surface of the crystal violet pectate (CVP) medium. Distinctive white-gray, circular bacterial colonies displaying smooth edges and characteristic cavities within the CVP medium emerged primarily due to their pectin-metabolizing capability and became prominent after a 3-day incubation period at 28°C. Thirty-one isolates with identical colony morphology were obtained from the CVP medium. To obtain a pure culture, colonies were continuously restreaked onto nutrient agar plates.

The soft rot symptoms caused by Pectobacterium aroidearum KNUB-05-21 on Zamioculcas zamiifolia in Emseong, Chun-gcheongbuk-do, Korea. (A) Infected Z. zamiifolia showed soft rot symptoms, including wilted appearance, water-soaked lesions, and stem collapse. (B) Infected stems turned green-brown. Arrow-heads indicate soft rot areas.
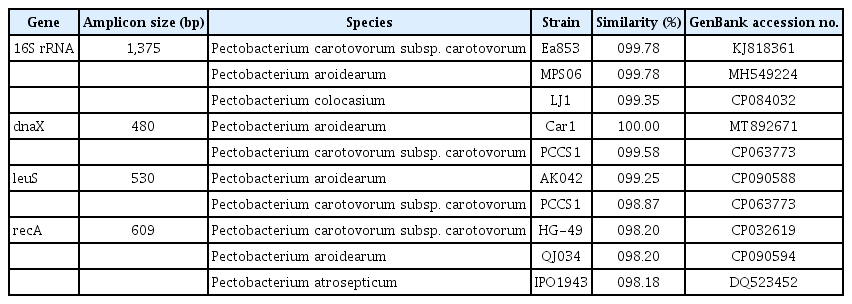
Using the HiGene Genomic DNA Prep Kit (Biofact, Daejeon, Korea), genomic DNA was extracted from all of isolated strains for molecular analysis. Amplification of the 16S rRNA gene of 31 isolated strains were carried out via polymerase chain reaction (PCR) using universal primers 9F/1512R, as outlined in Table 1. Afterward, the PCR products were separated through electrophoresis on a 1.5% agarose gel. The separated fragments were stained with ethidium bromide and visualized using ultraviolet light photography. Sequencing was performed using SolGent (Daejeon, Korea). Using the base sequence of the 16S rRNA region, it was determined that all strains isolated from the infected plant were identical (Supplementary Fig. 1). One of them, designed as KNUB-05-21, was randomly selected for further investigation. The 16S rRNA sequence of the strain was submitted to GenBank, acquiring the accession number LC777355. A BLAST search against the National Center for Biotechnology Information (NCBI) database unveiled a 99.35–99.78% similarity between the 16S rRNA region sequence of strain KNUB-05-21 and those of various strains within the Pectobacterium genus, including P. aroidearum, P. carotovorum subsp. carotovorum, and P. colocasium (Table 2).

Primers used to amplify the 16S rRNA region and three housekeeping genes (dnaX, leuS, and recA) in this study
Recently, Pectobacterium spp. were mostly identified and confirmed using the combination of 16S rRNA gene sequence analysis, multilocus sequence analysis (MLSA), and biochemical testing (Han et al., 2023; Park et al., 2022, 2023a, 2023b). In particular, the sequences of dnaX, leuS, and recA genes were successfully applied for phylogenetic analysis to revise the taxonomic status of several members of the genus Pectobacterium (Portier et al., 2019). The same approach was used in this study. For this purpose, the housekeeping gene sequences of 33 strains of Pectobacterium spp. and Dickeya solani were downloaded from GenBank (Supplementary Table 1). Using the primers in Table 1, the three housekeeping genes of strain KNUB-05-21 were amplified, and their sequences were submitted to GenBank with accession numbers LC777352, LC777353, and LC777354. MEGA version 7 was employed to conduct multiple sequence alignment (Kumar et al., 2016). The sequences of the obtained amplicons (dnaX, 480 bp; leuS, 530 bp; recA, 609 bp) were used for phylogenetic analysis (Table 1). Maximum likelihood analysis used the Kimura two-parameter model and the nearest-neighbor interchange heuristic search approach to construct the phylogenetic tree (Felsenstein, 1981). A monophyletic clade comprising strain KNUB-05-21 and several P. aroidearum strains (CFBP 1457, CFBP 2573, CFBP 6725, and CFBP 8737) with a high bootstrap value strongly suggested that they belong to the same species (Fig. 2).
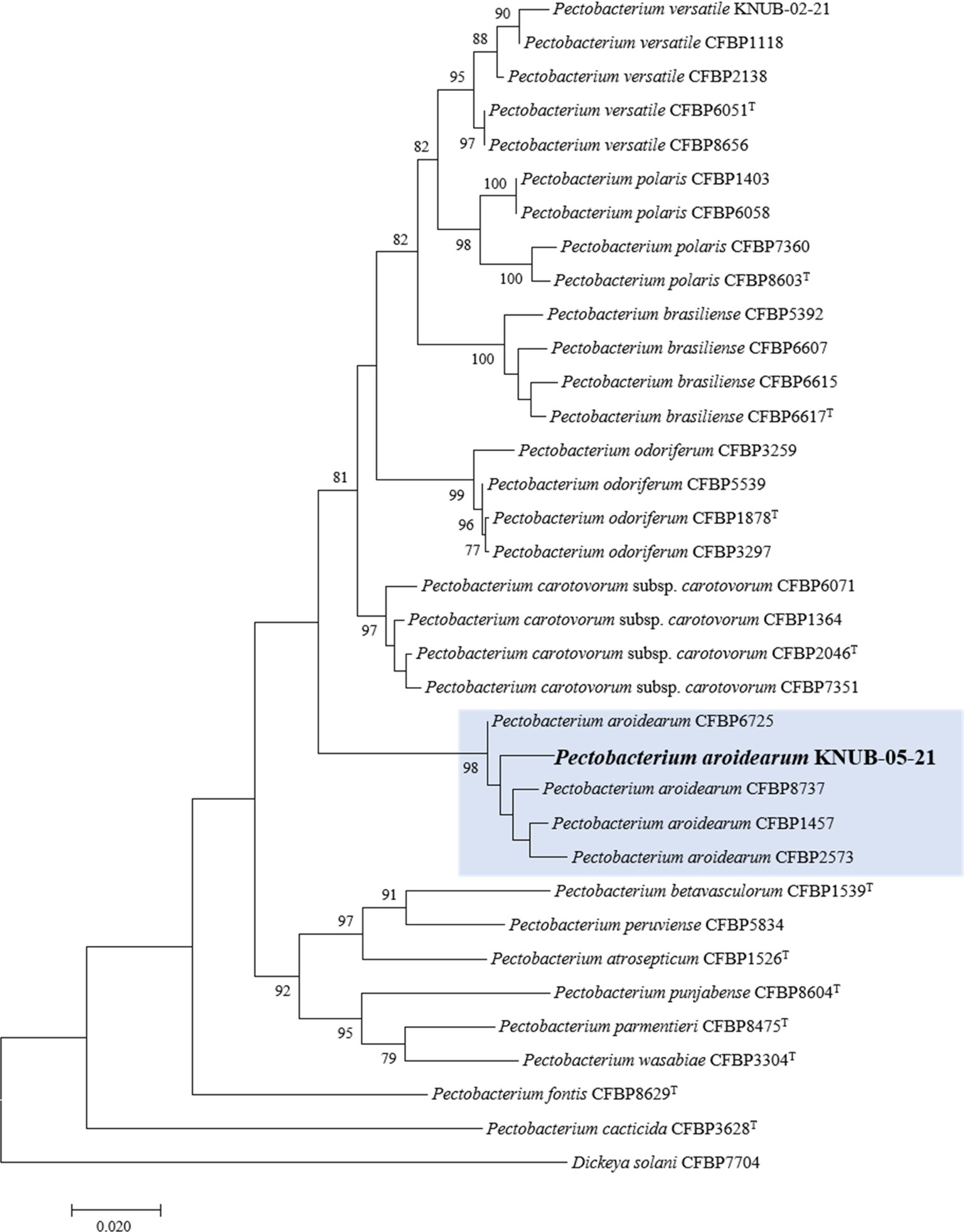
Maximum likelihood phylogenetic tree based on concatenated partial sequences of dnaX, leuS, and recA genes, showing the phylogenetic position of Pectobacterium aroidearum KNUB-05-21 among related species of the genus Pectobacterium. Bootstrap values (based on 1,000 replications) >70% are shown at branch points. The isolated strain is shown in bold. Dickeya solani CFBP 7704 was used as the out-group. The scale bar indicates 0.020 substitutions per nucleotide position.
Strain KNUB-05-21 was also investigated for compound utilization using the API ID 32 GN system (Biomérieux, Marcy l'Etoile, France) according to the manufacturer's instructions. Results showed that strain KNUB-05-21 was positive for N-acetylglucosamine, L-arabinose, D-glucose, inositol, D-mannitol, D-melibiose, L-rhamnose, L-serine, and sucrose but negative for L-alanine, glycogen, L-histidine, itaconic acid, lactic acid, D-maltose, L-proline, propionic acid, and valeric acid. Strain KNUB-05-21 exhibited almost all the characteristic features consistent with the type strain of P. aroidearum as described by Nabhan et al. (2013), except for its inability to metabolize D-glucose and lactic acid (Supplementary Table 2). This difference can be regarded as intraspecific variation among the strains belonging to P. aroidearum. Overall, findings from conventional biochemical tests were in accordance with molecular analysis results, thereby validating the classification of strain KNUB-05-21 as P. aroidearum.
Strain KNUB-05-21 was cultured in lysogeny broth (Difco, Sparks, MD, USA) at 28°C with shaking at 200 rpm to use for fulfillment of Koch's postulates. A 10 μl bacterial suspension was used to inoculate Z. zamiifolia plants. The inoculum concentration (1×108 cfu/ml) was measured at an optical density of 600 nm (OD600=0.8). Sterilized distilled water was used as a negative control. Z. zamiifolia plants were subjected to artificial wound induction followed by inoculation. Out of the four pots, three pots received a bacterial suspension treatment, while one pot served as the control. The pathogenic test was replicated three times. The inoculated plants were maintained in a glass cabinet with a controlled temperature (28°C) and high relative humidity of 80%. Within 24 hr after inoculation, soft rot symptoms were observed in the stems of Z. zamiifolia plants. The symptoms included a wilted appearance, water-soaked lesions, and eventual stem collapse (Fig. 3A, B). These symptoms were consistent with those observed in nurseries. Conversely, control Z. zamiifolia plants inoculated with sterile distilled water remained asymptomatic (Fig. 3C, D). Bacterial strains were subsequently reisolated from symptomatic plants and confirmed to be P. aroidearum by PCR and sequencing of the 16S rRNA region, thus fulfilling Koch's postulates.

Symptoms of soft rot disease in Zamioculcas zamiifolia caused by Pectobacterium aroidearum KNUB-05-21. (A) Soft rot symptoms on Z. zamiifolia infected by P. aroidearum KNUB-05-21. (B) The cross-section of the stems revealed dark-green to dark-brown soft rot in the pith region. (C) Sterilized water was used as a negative control. (D) Z. zamiifolia plants inoculated with sterile distilled water did not exhibit any symptoms.
Z. zamiifolia, a popular indoor plant known for its dark-green foliage and adaptability to low-light conditions, is cultivated in Korea. It effectively purifies indoor air by removing pollutants (Toabaita et al., 2016). However, it is susceptible to soft rot and foliar blight. However, the existing literature is deficient in studies concerning bacterial diseases in Z. zamiifolia. Soft rot is caused by bacteria belonging to the genus Pectobacterium. In particular, P. aroidearum was identified from various hosts. P. aroidearum, a Gram-negative, rod-shaped bacterium belonging to the Pectobacteriaceae family, is characterized by its facultative anaerobic nature, absence of spore formation, and possession of flagella that facilitate movement (Li et al., 2022). P. aroidearum causes bacterial soft rot in various plants (Mikiciński et al., 2023; Moraes et al., 2017, 2020; Tang et al., 2021; Wei et al., 2020; Xie et al., 2018; Xu et al., 2020). However, P. aroidearum was not previously reported as causing soft rot on Z. zamiifolia.
Through a comprehensive analysis that included 16S rRNA region sequencing, MLSA, and a detailed assessment of biochemical characteristics, the bacterial strain isolated in Korea from infected Z. zamiifolia, which displayed symptoms of soft rot disease, was identified as P. aroidearum. The pathogenicity of the isolated P. aroidearum was corroborated through successful inoculation experiments on Z. zamiifolia, thereby substantiating its role as the pathogen responsible for the observed soft rot. To date, P. aroidearum causing soft rot on Z. zamiifolia has not been officially reported in Korea. Recently, pathogen diagnostic methods for soft rot diseases have been developed for the sensitive and specific detection of target pathogens (Jin et al., 2022). However, compared to agricultural crops, there is a lack of comprehensive investigation regarding the survey of pathogens in indoor plants. Such endeavors are crucial for augmenting insights into the diverse array of potential diseases affecting indoor plants, consequently facilitating well-informed disease management strategies. Our findings provide a better understanding of P. aroidearum distribution in Korea and shed light on the relationship between Pectobacterium species and Z. zamiifolia soft rot. This understanding could potentially aid in developing effective control measures to prevent economic losses.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the Agriculture, Food and Rural Affairs Convergence Technologies Program for Educating Creative Global Leader Program funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (no. 321001-03).
Electronic Supplementary Material
Supplementary materials are available at Research in Plant Disease website (http://www.online-rpd.org/).