Phylogenetic and Recombination Analysis of Apple Stem Grooving Virus Isolates from Pears in Korea
Article information
Abstract
The apple stem grooving virus (ASGV) is one of the most harmful latent viruses infecting pear orchards worldwide. To examine the genetic diversity of ASGV in Korean pear orchards, the complete coat protein (CP) gene of five ASGV isolates collected from various regions were identified. The five Korean ASGV isolates showed 88–96% nucleotide identity with the 11 isolates worldwide occurring elsewhere in the world. Phylogenetic analysis of five isolates, as well as the previously sequenced isolates, indicated that the ASGV clusters had no correlation with the host or geographical regions of origin. Recombination analysis showed that one of the five Korean isolates is a recombinant, with a recombination site in the CP gene region (nt 532–708). This study is the first report of natural recombination within the CP gene of ASGV isolates from pears grown in Korea.
The pear is one of the most widely cultivated fruit trees in Korea. Pears are known to be susceptible to three major viruses: apple stem grooving virus (ASGV), apple stem pitting virus (ASPV), and apple chlorotic leaf spot virus (ACLSV). ASGV is one of the most important latent viruses infecting pear, with infection rates as high as 70% reported in many commercial pear cultivars in Korea (Cho et al., 2010; Nam and Kim, 1994; Yoon et al., 2014). ASGV belongs to the genus Capillovirus in the family Betaflexiviridae (Adams et al., 2012; Lefkowitz et al., 2018). In addition, ASGV is known to be trans-mitted by grafting, although, no biological vector is known (Adams et al., 2012). It also infects citrus fruits, apricots, and lilies (Ohira et al., 1994; Takahashi et al., 1990), and leads to the development of long grooves on the woody stem, black necrotic leaf spots, and graft union necrosis of sensitive cultivars (Hong et al., 1985; Welsh and van der Meer, 1989). RNA recombination is the exchange of genetic information between RNA viruses with non-segmented RNAs, and it is one of the major factors affecting RNA genome variability during viral replication (García-Arenal et al., 2001). Recombination events resulting in the emergence of new isolates of plant RNA viruses can be of selective advantage under certain cir-cumstances, which could help host adaptation and influence RNA virus evolution. It can also help in identifying recombination hot spots, which can generate information useful for devising crop protection strategies (Holmes, 2009). There are few studies reporting the phylogenetic and recombination events in the genome of ASGV or other pear infecting viruses (Dhir et al., 2013; Yoon et al., 2014). Recently, Chen et al. (2014) analyzed the phylogenetic relationships and recombination events in the genome of ASGV isolates from apples in China; however, no molecular characterization and recombination tests based on the genome sequences of ASGV isolates from pears are lacking. To examine the genetic variability of ASGV spread in pear trees in Korea, we randomly collected 158 leaf samples that exhibited leaf chlorosis or necrotic spots from 34 pear orchards in five intensive pear growing areas (Chonan, Naju, Namyangju, Sangju, and Ulsan) from April to October, from 2018 to 2020.
To detect ASGV using reverse transcription polymerase chain reaction (RT-PCR), total RNA was extracted from one leaf from each tree using IQeasy, plus the plant RNA extraction Mini Kit (iNtRON, Seongnam, Korea) according to the manufacturer's recommendations. One-step RT-PCR was performed in a total volume of 20 μl containing 1 μl of total RNA, 1 μl of each primer (10 pM), 10 μl of SuPrimeScript RT-PCR Premix (GeNet Bio, Daejeon, Korea), and 7 μl of DEPC water. The forward primer sequence (5′-ATGAGTTTG-GAAGACGTGCTTCAA-3′) and the reverse primer sequence (5′-CTAACCCTCCAGTTCCAAGTTACT-3′) were used to am-plify the complete coat protein (CP) coding sequences (Shim et al., 2004). The thermal conditions were as follows: stage 1, 50°C for 30 min; stage 2, 95°C for 5 min; stage 3, 35 cycles of 95°C for 30 sec, 58°C for 30 sec and 72°C for 60 sec; and stage 4, 72°C for 5 min. The RT-PCR products were determined after cloning into a pCR2.1-TOPO TA vector (Invitrogen, Grand Island, NY, USA), and the nucleotide sequences of the inserts were determined. Five ASGV CP isolates (MG682506 from Sangju; MG682507 from Namyangju; MG682508 from Ul-san; MG682509 from Cheonan; MG682510 from Naju) were identified, and the CP gene nucleotide sequences from the isolates were deposited in NCBI (Kim et al., 2018). The ASGV CP gene is 714 nucleotides in length. To compare nucleotide sequence identity, five CP gene sequences of ASGV isolates from pears in Korea were aligned using ClustalW implemented in Bio-Edit with those of 11 isolates occurring in other countries (KR606324, LC086300, KF735124, LC184610, KX988001, KR815877, GQ330294, LN90143, JX885582, JN792491, and KX668488) (Table 1). Pairwise distances were calculated using the PASC algorithm, available in the Gen-Bank database (Bao et al., 2012). The complete nt sequence identity values of the CP gene in the isolates ranged from 89.0% to 98.0% (Fig. 1A). Among the five Korean ASGV isolates, sequence identity was the highest (93%) between the isolates MG682506 and MG682509 and the lowest (90%) between isolates MG682510 and 682507. The N-terminal region of the viral CP gene was a little variable, while the C-terminal region was highly conserved (Supplementary Fig. 1). MG682506, MG682507, MG682508, MG682509, and MG682510 shared 89–97%, 88–91%, 88–96%, 90–93%, and 90–97% identity with the other 15 isolates, respectively, in-dicating that the CP gene, as reported previously (Chen et al., 2014), is the most conserved. To determine the genetic relationships among the 16 ASGV isolates, we aligned the nucleotide sequences and constructed a phylogenetic tree using the complete CP coding sequences. The phylogenetic analysis was performed with a neighbor-joining algorithm using the Kimura 2-parameter methods with 1,000 bootstrap replicates, as implemented in the Molecular Evolutionary Genetics Analysis tool (MEGA 5.05) (Tamura et al., 2011). The ASGV isolate MG682510 grouped within the same clade as isolates from the Korea apple and pear, whereas the ASGV isolates MG682506, MG682507, MG682509, and MG682508 were placed as different clade, respectively (Fig. 1B). The phylogenetic study of the global ASGV isolates, based on CP coding sequences, showed clear separation of the ASGV isolates from all other isolates, with a high bootstrap value. This indicates that the ASGV clusters have no correlation with the host or the geographical regions of origin. A similar pattern has been observed in a previous study wherein 54 ASGV isolates, which collected from around the world, were separat-ed into two groups, and no host grouping was seen in their phylogenetic study (Liu et al., 2013). Moreover, to elucidate recombination network among the examined ASGV isolates, we generated a splitstree using SplitsTree 4.11 (Huson, 1998). For network creation, a Clustal format file was generated using the ClustalX2 program package (Larkin et al., 2007), based on the aligned ASGV CP coding sequences. The splits network showed that our isolates branched separately from each other (Fig. 2). The reticulate topologies, however, also indicated that a potential recombination event might have occurred among the ASGV isolates collected from the other countries.
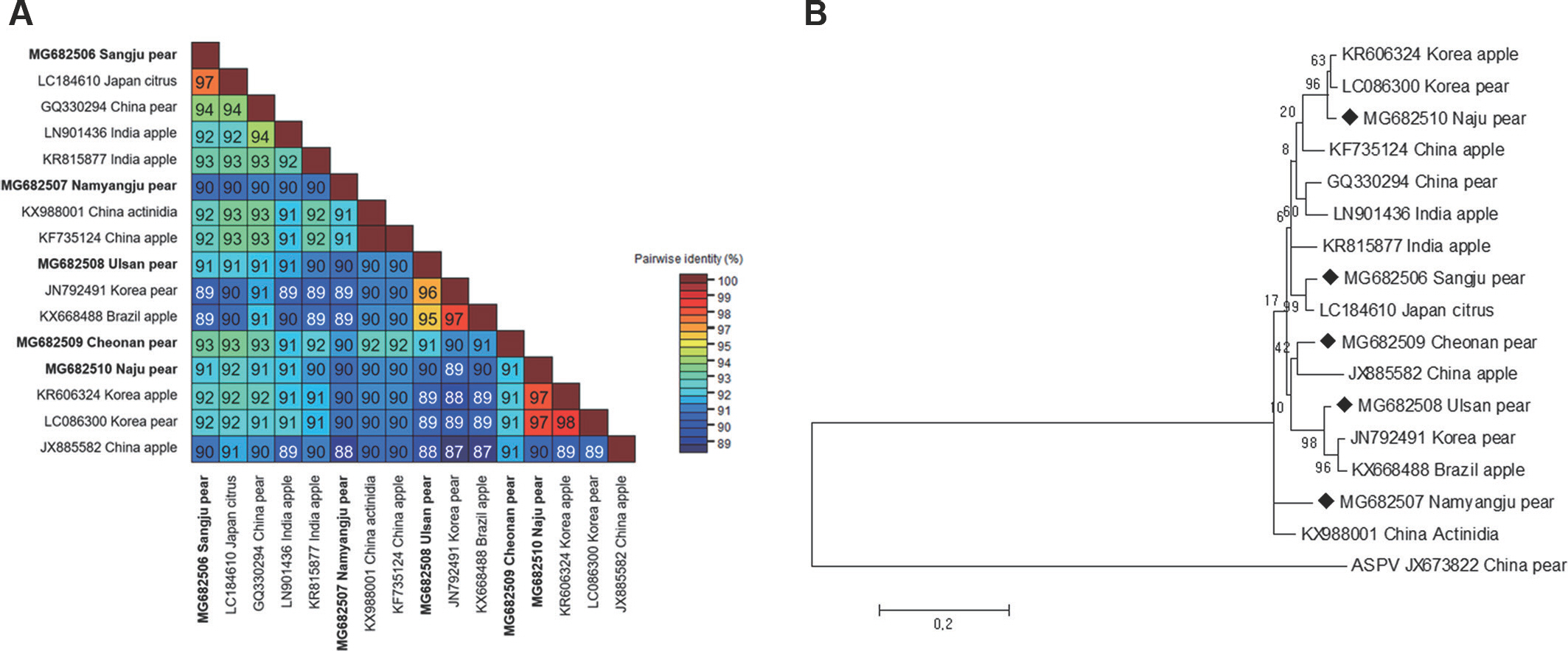
Pairwise comparisons and phylogenetic tree of the complete coat protein (CP) coding sequence of apple stem grooving virus (ASGV) isolates. Information on the accession number, geographic location, and host plant for each ASGV isolate is listed by order of the taxon names. (A) Each number represents pairwise identities between the corresponding isolates. (B) Phylogenetic tree constructed using the neighbor-joining method based on the complete CP gene nucleotide sequence of ASGV isolates obtained from GenBank. Apple stem pitting virus (ASPV, Foveavirus, JX673822) was used as an outgroup for each analysis. Bootstrap values along the branches are supported by 1,000 replicates. The scale bar indicates the number of substitutions per residue.
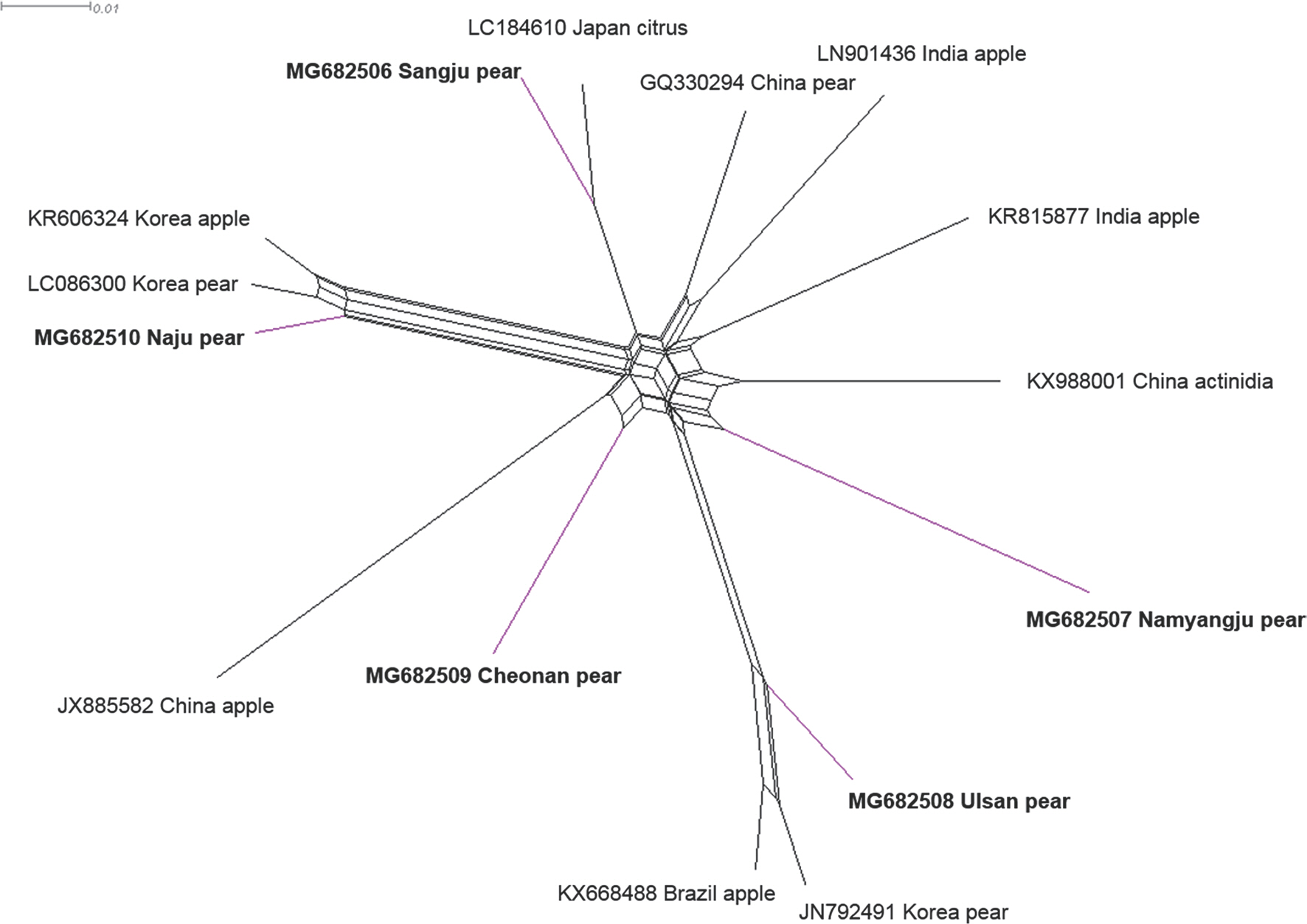
Phylogenetic networks of complete coat protein coding sequences of apple stem grooving virus (ASGV), generated using Splitstree. Blue color indicates that five different regions of Korean ASGV isolates from pears. The networks were created with SplitsTree 4.11 using the uncorrected P characters transformation.
To further determine the putative recombination signals observed by the network analysis, we conducted a recombination detection analysis using the RDP4 software package (Martin et al., 2005b), which contains seven different programs: RDP (Martin and Rybicki, 2000), GENECONV (Padidam et al., 1999), BootScan (Martin et al., 2005a), MaxChi (Smith, 1992), Chimaera (Posada and Crandall, 2001), SiScan (Gibbs et al., 2000), and 3Seq (Boni et al., 2007). A P-value cut-off of 0.05 was estimated according to the default parameters, except for the options of linear sequence and disentangling overlapping signals (Cordin et al., 2012; Li et al., 2013). The 16 complete CP coding sequences were processed and tested for recombination. We detected one recombination event by six different methods (Fig. 3). The minor parent, major parent, beginning breakpoint, ending breakpoint, programs detecting the event and the corresponding P-value for a recombinant is listed in Table 1. The event is an intrastrain recombination event at the 3′ terminus of the CP gene. The analysis showed that an isolate from Ulsan (MG682508) is a recombinant, composed of a 714 nt fragment of the CP component, between the minor parent isolate from Sangju (MG682506) incorporated into the major parent isolate from pear in Korea (JN792491) (Fig. 3A). The exact positions of the beginning and ending break points varied, depending on the isolate sequence and algorithm used in the analysis (Table 2). The beginning and ending breakpoints located from 532 nt to 708 nt. To further confirm the potential recombination event, we examined pairwise identity using the RDP plot. Identity plots revealed that recombination between paren-tal isolates was evident for recombination event, where the upward peak of the minor parent plots were coherent with the breakpoint stretches identified by the RDP4 program (Fig. 3B). Clearly, a predicted recombination event could ac-count for the close phylogenetic relationships among some isolates (Fig. 3B). Although four recombinants have been reported based on the complete ASGV genome sequences, a recombinant with recombination in the CP coding gene has not been reported (Chen et al., 2014). Recombination events in the coding regions of the genome are likely to affect the adaptations of recombinants to various environ-ments. Recombination events in the CP genes, especially, play important roles in the viral infection and life-cycle (Chare and Holmes, 2006). Taken together, these analyses indicate the occurrence of genetic recombination in ASGV isolates; the results also establish the recombinant identified to be the first CP gene recombinant of ASGV isolates from pear in Korea.
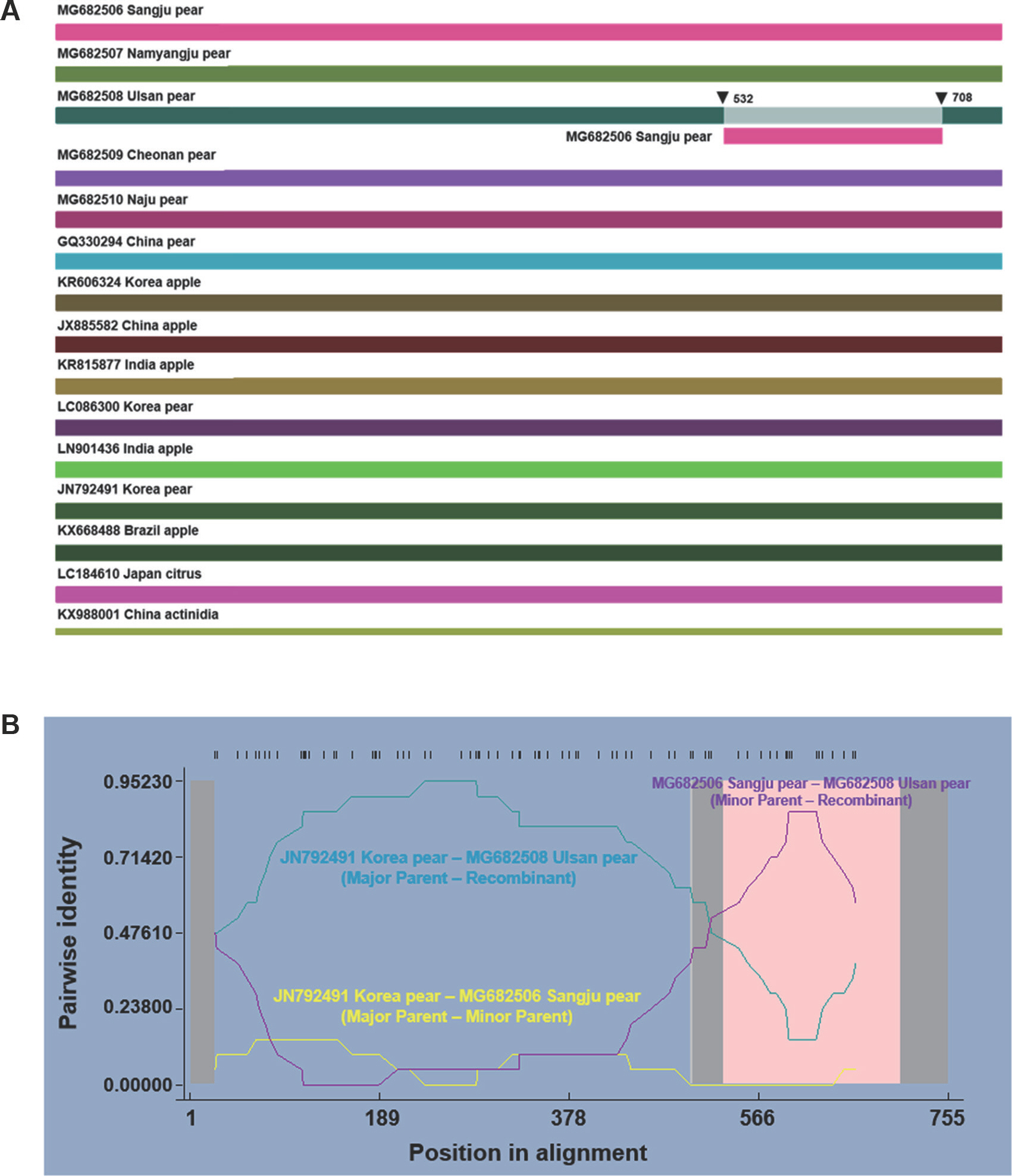
Schematic representation of one recombination event found in the coat protein (CP) gene among apple stem grooving virus (ASGV) isolates, using RDP4 analysis. (A) One recombination event was detected in the CP sequences from MG682508 (Ulsan) of Korea. (B) RDP evidence for the recombination between ASGV isolates. Both the analyses indicate that MG682508 is a recombinant, deriving genome fragment from JN792491 and MG682506, as the major and minor parents, respectively. The pink zone represents the region of recombination between the genomes.
Here, we identified the genetic diversity and conducted recombination analysis of ASGV isolates, originating from pear orchards located in areas of intensive pear cultivation in Korea. Recently, several studies have shown that ASGV has a wide natural host ranges that includes herbaceous plants (Chung et al., 2022; Lee et al., 2023). These natural host preference or adaptation of ASGV may be driven by micro-evolution within translational selection forces, especially host codon usage frequency (Kim et al., 2021). To our knowledge, this is the first analysis of recombination based on CP gene sequences of ASGV isolates from pear in Korea. Although no recombination hotspots were found, recombination events show a critical role in the evolution of the RNA virus (Lian et al., 2013; Negi et al., 2010). In addition, genetic recombination has also been frequently reported in members of Betaflexiviridae (Alabi et al., 2014; Chen et al., 2014; Yoon et al., 2014). The fact that there are phylogenetic clusters, with no correlation between the host or geographical regions of origin and recombinant events in isolates from different host species and countries, is possibly due to vegetative propagation and trades of propagation materials between countries. Given that recombination was identified in the CP coding region of the ASGV genome, there is requirement for further analysis of whole-genome recombination in ASGV isolates from pears in Korea, from which would be possible to understand the influence of the host species on adaptation through recombination.
Electronic Supplementary Material
Supplementary materials are available at Research in Plant Disease website (http://www.online-rpd.org/).
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through (Agri-Bioindustry Technology Development Program), funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No. 317006-04-2-HD030).


