Chrysanthemum Chlorotic Mottle Viroid-Mediated Trafficking of Foreign mRNA into Chloroplasts
Article information
Abstract
Chrysanthemum chlorotic mottle viroid (CChMVd) fused to the leader sequence of a reporter gene (mRFP) expressed transiently in agroinfiltrated Nicotiana benthamiana, was used to show that CChMVd can traffic into chloroplasts, thought to be the site of its replication. Fluorescence from mRFP was detected in chloroplasts, but only if the viroid transcription fusions were present, either from the full-length 400-nt CChMVd, or each of two partial fragments (nucleotides 125 to 2 and 231 to 372). The mRFP and its mRNA were detected by western blotting and RT-PCR, respectively, in tissue extracts of plants infiltrated by each fusion construct. Isolated chloroplasts were shown by RT-PCR to contain the RNA sequences of both CChMVd and mRFP, if both were present, but not the mRFP sequence in the absence of the viroid sequences. The results suggest that RNA trafficking was probably due to an RNA structure, and not a particular sequence, as discussed.
Introduction
Two important pathogens of chrysanthemum (Dendrathema grandiflorum) in Korea are the viroids chrysanthemum stunt viroid and chrysanthemum chlorotic mottle viroid (CChMVd) (Chung et al., 2005, 2006). CChMVd can also give a symptomless infection, depending on sequence variants and the chrysanthemum cultivar (Chung et al., 2006; Yamamoto and Sano, 2006). Due largely to the low titer of CChMVd in infected plants, the molecular biology of its interactions with its host have not been as well-studied as for other viroid members of the family Avsunviroidae, which include avocado sunblotch viroid (ASBVd), eggplant latent viroid (ELVd) and peach latent mosaic viroid (PLMVd) (Ding, 2009; Flores et al., 2005, 2014; Palukaitis, 2014). ASBVd (Bonfiglioli et al., 1994; Lima et al., 1994; Navarro et al., 1999, 2000), and PLMVd (Bussière et al., 1999) have been shown to replicate in chloroplasts, while ELVd has been shown to traffic mRNA into chloroplasts for expression of the encoded protein (Gómez and Pallas, 2010a, 2012) and to be ligated by a chloroplast isoform of tRNA ligase (Nohales et al., 2012); however, there are no studies demonstrating that CChMVd can enter chloroplasts. Therefore, to establish whether CChMVd can enter chloroplasts, we used an analysis system similar to that described by Gómez and Pallas 2012) for ELVd.
The full-length CChMVd sequence [Korean isolate SSHA6 (Chung et al., 2006)], from nucleotides 1-400, was introduced into an agroinflitration plasmid upstream of a reporter gene, encoding the monomer red fluorescent protein (mRFP), in the plasmid pROK2-mRFP, using the Gateway System (In-Vitrogen, USA) to create pCChMVd-FL-ROK2-mRFP (Fig. 1A, 1B). Both plasmids were transformed into Agrobacterium tumefaciens, which was then used to agroinfiltrate leaves of Nicotiana benthamiana as described (Canto et al., 2002). Five days after infiltration, the plants were examined by confocal microcopy to detect the presence of fluorescence due to expression of the reporter gene mRFP, which has an emission spectrum optimum different from that of chlorophyll autofluorescence (Campbell et al., 2002). Fluorescence was observed in the cytoplasm and in chloroplasts, but it was weaker for the construct containing the full-length CChMVd sequence (pCChMVd-FL-ROK2-mRFP) (Fig. 2A) than for the control construct (pROK2-mRFP), which did not contain any viroid sequences (Fig. 2A). This probably was due to a reduction in the efficiency of translation caused by the presence of the highly structured viroid sequence upstream of the reporter gene and also was observed with ELVd (Gómez and Pallas, 2010a, 2010b, 2012). Also, while fluorescence due to the green fluorescent protein (GFP) produced from the ELVd-fused to the GFP mRNA was only localized in the chloroplasts, the fluorescence produced from expression of pCChMVd-FL-ROK2-mRFP was present mostly in the chloroplasts, but also some remained in the cytoplasm (Fig. 2A). Expression of the mRFP protein in the cells was confirmed by western blot analysis of proteins extracted from the infiltrated tissues, with an antiserum to RFP protein (Abcam, USA) (Fig. 2B), done as described by Canto and Palukaitis (1999).

Constructs of Agrobacterium plasmids that express mRFP after agroinfiltration into Nicotiana benthamiana leaves and secondary structure of CChMVd. (A) pROK2-mRFP alone, (B) pROK2-mRFP containing the full-length CChMVd sequence (400 bp), (C) pROK2-mRFP containing the Partial-I insert (278 bp; from nucleotides 125-400/1-2), (D) pROK-mRFP containing the Partial-II insert (142 bp; from nucleotides 231-372). (E) Secondary structure of CChMVd based on the alternative structure shown determined by Giguère et al. (2014), showing nucleotide positions of the termini of the CChMVd sequences cloned into the plasmids in (B-D).
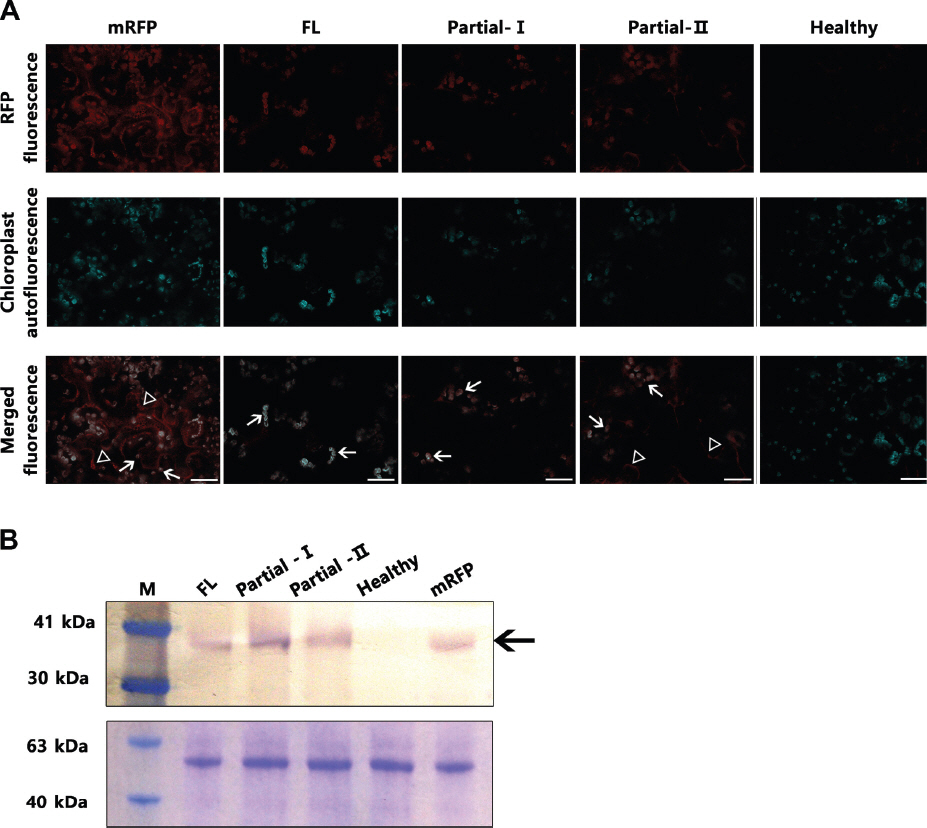
Expression of mRFP and localization after agroinfiltration into N. benthamiana leaves. (A) mRFP expression in agroinfiltrated leaves analyzed using a confocal laser scanning microscope (Leica, Germany) with excitation at 570 nm and emission at 650 nm. The constructs agroinfiltrated from Fig. 1 are described along the top (FL=mRFP vector with Full-length CChMVd; mRFP vector with Partial-I=ChCMVd sequences 125-2; Partial-II =mRFP vector with CChMVd sequences 231-372; mRFP =mRFP vector with no viroid sequences; healthy=no agroinfiltration). The images show fluorescence due to mRFP (top row), autofluorescence of chloroplasts (middle row), or both merged (bottom row). Autofluorescence of chloroplasts was detected at 670 nm (excitation) and 750 nm (emission). Some fluorescent chloroplasts are indicated (arrows), as are fluorescent membranous structures (arrowheads). Scale bars=20 μm. (B) Western blot assay for confirmation of expression of mRFP after agroinfiltration (upper panel), and duplicate Coomassie blue-stained gel showing plants proteins used as a loading control (lower panel). Proteins were extracted from agroinfiltrated tissues and subjected to SDS-polyacrylamide gel elctrophoresis, transfer to a nitrocellulose membrane, and probing with a primary mouse antiserum against RFP and a secondary anti-mouse serum conjugated to alkaline phosphate (Promega, USA), followed by a colorimetric detection assay (Promega). The mol. wt. of selected protein markers are shown on the left and the position of mRFP is shown by an arrow on the right. The expected mobility of the mRFP is 34 kDa.
To confirm the presence of the viroid sequence and the mRFP mRNA in the chloroplasts, the latter were isolated from agroinfiltrated leaves using the method described by Gómez and Pallas (2010a). Briefly, 10 g of tissue was homogenized in an isotonic buffer, and filtered through Miracloth. The filtrate was subjected to centrifugation at 1500 g for 10 min and the pellet was resuspended in a buffer without polyvinylpyrrolidone, loaded onto a Percoll step gradient (15%–35%–50%) and centrifuged at 8000 g for 20 min. The intact chloroplasts were recovered from the interface between 35% and 50% Percoll, were washed in isolation buffer, and were collected by centrifugation (1000 g for 5 min). The chloroplasts were resuspended, viewed under an epifluorescent microscope (Fig. 3) and the chloroplast RNAs were extracted. Extracted RNAs were analyzed for the presence of both CChMVd and mRFP RNA sequences, as well for chloroplast 16S rRNA and for the absence of cytoplasmic 18S rRNA, by the reverse-transcription polymerase chain reaction (RT-PCR). Total RNAs were extracted from these chloroplasts using the IQeasyTM Extraction Mini Kit (Intron, Korea) according to the manufacturer’s instructions. The concentration of the recovered RNAs was determined using the Nanodrop 2000 (Thermo, USA). Complementary DNAs(cDNAs) were synthesized from the RNAs, using RevertAid Reverse transcriptase (Thermo Scientific, USA) as per the manufacturer’s instructions, using the Random hexamer (Qiagen, Germany). PCR amplification of the cDNAs was done as described (Medlin et al., 1988; Weisburg et al., 1991), using TaKaRa Ex Taq (Takara, Japan) polymerase, according to the manufacturer’s instructions, and the above primers as well as the following primers: CChMVd-U 5’- TCCTTTGGAGTCCATTTTTCTC- 3’, CChMVd-L 5’-TCCTTTGGAGTGGACTAAGA- 3’; mRFP gene-U 5’-CCAAGCTGAAGGTGACCAAG- 3’, mRFP gene-L 5’-ATGGTGTAGTCCTCGTGTTGG-3’; 16S rRNA-U 5’-AGAGTTTGATCMTGGCTCAG-3’, 16S rRNAL 5’-CGGTTACCTTGTTACGACTT-3’; and 18S rRNA-U 5’-AACCTGGTTGATCCTGCCAGT-3’, 18S rRNA-L 5’-GGCACCAGACTTGCCCTC- 3’, respectively. The PCR products were analyzed by electrophoresis in 1.2% agarose gels followed by staining with ethidium bromide and photography under UV. First, these analyses demonstrated the expression of the RNA from the above plasmids infiltrated into whole leaves contained either the mRFP sequence or the CChMVd and mRFP sequences, and both contained 16S and 18S rRNAs (Fig. 4, right). Second, the analyses showed the purity of the chloroplast preparations, by the presence of chloroplast 16S rRNA sequences and the absence of contaminating cytoplasmic 18S rRNA sequences in the same samples (Fig. 4, left). And third, the analyses also confirmed the presence of CChMVd and mRFP RNA sequences in the RNAs extracted from isolated chloroplasts obtained from plants agroinfiltrated with bacteria containing pCChMVd-FL-ROK2-mRFP, but absent in the RNAs extracted from the isolated chloroplasts obtained from plants agroinfiltrated with bacteria containing pROK2-mRFP (Fig. 4, left). Thus, the CChMVd inserted into the leader sequence of the gene encoding the mRFP reporter was able to traffic the transiently expressed mRNA into the chloroplast, similarly to what had been shown with ELVd, although apparently less efficiently (Gómez and Pallas, 2010a, 2012).

Chloroplasts isolated from agroinfiltrated plants expressing the construct pCChMVd-FL-ROK2-mRFP. Chloroplasts were imaged using an epifluorescence microscope (Zeiss, Germany) at 400X, under visible light (upper), under UV light (center) and with both images merged (lower). The arrow points to a mass of chloroplasts showing fluorescence due to mRFP, while arrowheads point to chloroplasts seen in visible light, but not under UV light. Scale bar=10 μm.
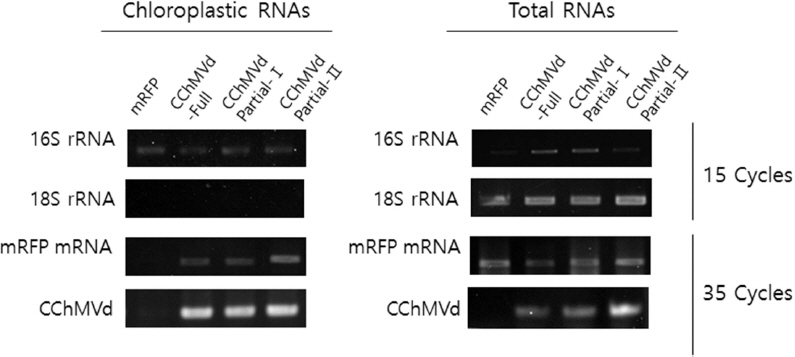
RT-PCR analysis of total RNAs and isolated chloroplast RNAs from tissues infiltrated with bacteria harboring mRFP expressing plasmids. mRFP transcripts extracted from the above samples were subjected to RT-PCR using primers specific to the mRFP gene, 18S cytoplasmic rRNA, 16S chloroplast rRNA, and CChMVd, for the cycles indicated. Since chloroplasts contain 16S rRNA and do not contain cytoplasmic 18S rRNA, the absence of the 18S rRNA from the chloroplast preparation is a measure of the purity of the chloroplasts.
To determine whether the whole CChMVd sequence was needed to traffic the reporter gene mRNA into the chloroplast, two fragments of the CChMVd genome, representing nucleotides 125 (through 400) to 2 (Partial-I; pCChMVd-PartIROK2-mRFP) and from nucleotides 231 to 372 (Partial-II; pCChMVd-PartII-ROK2-mRFP) were inserted into the leader sequence of the mRFP gene in pROK2-mRFP (Fig. 1C, 1D). These constructs were then transiently expressed in N. benthamiana leaves as for the full-length construct and the fluorescence was examined at 5 days after infiltration. As can be seen on Fig. 2A, Fig. 2 both constructs expressed mRFP and both showed partition of fluorescence between the cytoplasm and the chloroplasts. The mRFP in cells agroinfected by bacteria expressing pCChMVd-partI-ROK2-mRFP was present mostly in the chloroplasts, but also present in the cytoplasm. By contrast, the mRFP derived from pCChMVd-partII-ROK2-mRFP was present mostly in the cytoplasm, somewhat similar to that of mRFP derived from pROK-mRFP (Fig. 2A), indicating that the mRFP mRNA containing the shorter CChMVd-derived sequence (nucleotides 321-372) did not traffic as efficiently to the chloroplast as the mRFP mRNA derived from the other constructs containing CChMVd sequences. Proteins extracted from plants infiltrated with either construct also showed the presence of mRFP protein (Fig. 2B), while the presence of CChMVd sequences and the mRFP sequences in RNAs extracted from the infiltrated plants was shown in RT-PCR assays (Fig. 4, right). In addition, RNAs extracted from chloroplasts isolated from plants infiltrated with bacteria containing each of these constructs showed the presence of CChMVd sequence and the mRFP sequences by RT-PCR, as well as the presence of 16S rRNA sequences, but not 18S rRNA sequences (Fig. 4, left).
These data showed that CChMVd fragments, containing either the right two-thirds of the viroid structure (Partial-I) or the central third (Partial-II), containing six or three of the six stem-loop structures, respectively, from the right six-way junction present (see Fig. 1E) (Giguère et al., 2014), was able to facilitate RNA trafficking into chloroplasts, although to different extents, with the latter being less efficient. In the case of ELVd, the region that was able to traffic fused mRNA for GFP into the chloroplasts was the left one-third of the RNA structure (nucleotides 52-150, containing two stem-loop structures of the left three-way junction), without the rest of the molecule, although not as efficiently as the full-length molecule (Gómez and Pallas, 2010b, 2012). Since ELVd and CChMVd do not contain any sequence similarity, beyond the limited sequences (11 of 13 nt split into four parts) involved in hammerhead ribozymes (Giguère et al., 2014), then the ability of both RNAs to traffic RNAs into chloroplasts most likely resides in their structures. Since CChMVd, ELVd and PLMVd all contain multiple branch junctions, but ASBVd does not, according to the current structural models (Giguère et al., 2014), then either these branching-structure aspects are not necessary for trafficking into chloroplasts, or they are and ASBVd adopts a different structure (with or without the help of binding proteins) to facilitate its movement into chloroplasts. In either event, CChMVd enters the chloroplast, which together with the evidence that it can be ligated into a circle by the chloroplast isoform of tRNA ligase in vitro (Nohales et al., 2012) supports the model suggesting that CChMVd, like other members of the Avsunviroidae family replicates in chloroplasts (Flores et al., 2014).
Acknowledgement
This study was supported by grant number PJ011309032017 from the Next Generation BioGreen21 Program of the Rural Development Administration, Republic of Korea.
References
Notes
Conflicts of Interest
The authors declare that they have no competing and commercial interests in this work.