Screening of Pumpkin (Cucurbita spp.) Germplasm for Resistance to Powdery Mildew at Various Stages of Seedlings Growth
Article information
Abstract
Powdery mildew (Podosphaera xanthii) causes severe damage to pumpkin crops grown in open fields and plastic house. Initially, we used ten accessions of pumpkin species; Cucurbita pepo (3), C. maxima (2), C. moschata (2), and C. argyrosperma (3) to study the disease progress in different stages of seedling development. Commercial pumpkin varieties were used as susceptible host for inoculum management and young seedlings were inoculated by dusting powdery mildew spores on the cotyledons, 1-true-leaf and 2-true-leaf seedling stages. Based on initial study, we further evaluated 218 pumpkinaccessions for their resistance to powdery mildew in different seedling stages under plastic house. Area under disease progress curve (AUDPC) and relative AUDPC (rAUDPC) was higher in cotyledonary and 1-true-leaf than 2-true-leaf stage. Seedlings at cotyledons and 1-true-leaf seedling stage displayed more susceptibility to powdery mildew. Based on evaluation of 2-true-leaf stage, IT 110859 and IT 278459 from C. pepo and C. argyrosperma identified as resistant (<0.2). Of the 228 pumpkin accessions, 21 (9.2%) pumpkin accessions consisting of C. pepo (2), C. maxima (5), C. moschata (13), and C. argyrosperma (1) exhibited intermediate resistance (<0.4) to powdery mildew and these accessions are useful to growers for its rational management.
Introduction
Powdery mildew is one of the world’s most widespread and damaging diseases of cucurbits (Kousik et al., 2008). It is caused by several species of the Erysiphaceae including Podosphaera xanthii (syn. Sphaerotheca fulginea) (McGrath and Thomas, 1996; Thomas et al., 1984). This disease acts as a sink for plant photosynthates causing reduction in plant growth, premature foliage loss, and consequently a reduction in yield. The yield loss is proportional to the severity of the disease and the length of time that plants have been infected (Mossler and Nesheim, 2005). It also results the reduced vigor of the seedlings and death of the seedlings in some instances (Kousik et al., 2008).
In Korea, S. fuliginea is the most prevalent pathogen of powdery mildew in cucurbits (Shin, 2000). To date, seven races of P. xanthii (syn. S. fuliginea) have been identified using melon (Cucumis melo L.) differentials (Cohen et al., 2004; Pitrat et al., 1998; Thomas et al., 1984). Variability within races 1 and 2 in P. xanthii populations was described using 32 melon cultigens with the potential for the existence of 28 races (McCreight, 2006). Physiological races of this pathogen have not been classified for other cucurbits because of the lack of differentials and fully resistant germplasm (Cohen et al., 2004). A powdery mildew isolate from one cucurbit can infect all other tested cucurbit spp. (Hammett, 1977) and Cohen et al. (2004) has provided detail description of the cucurbit powdery mildew pathogen and race classification system. In the past, screening and selection studies against powdery mildew disease were undertaken in the accessions of watermelon (Thomas et al., 2005), cucumber (Block and Reitsma, 2005), bottle gourd (Kousik et al., 2008) and Cucurbita sp. (Kim et al., 2014). Though Korean varieties of Cucurbita moschata have good quality fruit, these are susceptible to powdery mildew (Cho et al., 2003). Powdery mildew is a limiting factor for pumpkin production and it spreads throughout the field at the end of growing season (Cohen et al., 2000). Screening of pumpkin germplasm for powdery mildew is the best way in developing new variety to prevent this disease.
National Agrobiodiversity Center of Rural Development Administration, Korea has introduced many accessions of pumpkin from abroad in recent years. However, little is known about the extent of powdery mildew resistance in these collections. Disease resistance screening in introduced germplasm is important to get resistant and tolerant germplasm for breeding a new cultivar and controlling powdery mildew in field and greenhouse-grown pumpkins. Cotyledonary stage is appropriate for screening powdery mildew in C. melo (Cohen and Eyal, 1995) and screening for resistance at young plants stage is common and accurate method in greenhouse condition (Cohen and Cohen, 1986). Despite the different screening methods reported in different conditions, the right stage of screening has not been studied yet under plastic house condition and disease severity might also be varied in different developmental stages of seedlings. Therefore, the objectives of this study were to study the disease progress curve in different developmental stages of seedling in Cucurbita species and, to screen a large number of pumpkin accessions in different seedlings stages against powdery mildew under plastic house condition.
Materials and Methods
Host management
Seeds of five pumpkin commercial varieties (Nong-Woo Hae, Kolthan-Jee, Thanpham, Bokzhuphus, Hoekjong Pumpkin) were purchased from market and sown in February 25, 2015. Seedlings were transplanted at 60 cm apart at both sides of the plastic house in April 8, 2015 and used as susceptible hosts for powdery mildew.
Identification of P. xanthii and inoculum preparation
Pumpkin leaves infected with powdery mildew were collected from farmer’s fields (Jeonju, Korea) in April 21, 2015 for microscopic observation of the pathogen. The conidia observed under light microscope (Leica DM5500B; Leica, Wetzlar, Germany) were oidium type and conidiospore with cylindrical fibrosin bodies of conidia which was identified to be P. xanthii. Earlier researchers (McGrath et al., 1996; Shin, 2000) were confirmed the pathogen to be P. xanthii, caused powdery mildew in pumpkin based on this similar microscopic examination. Then, artificial inoculation of host plants seedlings were performed by manually through dusting the sporulated leaves and P. xanthii isolates in host plants were kept for 60 days in plastic house.
Plant materials
Initially, seeds of 10 accessions constituting three accessions (IT 278455, IT 278462, IT 278463) from Cucurbita pepo, two accessions (IT 278453 and IT 278471) from C. maxima, two accessions (IT 278454 and IT 278474) from C. moschata and three accessions (IT 278459, IT 278460, and IT 278464) from C. argyrosperma were sown in plug trays containing horticultural soil (Bio-media Co., Ltd., Seoul, Korea) in June 1, 2015 to study the disease progress in different stages of seedling. Each accession had three seedlings and repeated six times. Pathogen was inoculated on cotyledons through host plants netted above the test seedlings and naturally dropped the spore from the susceptible host plants on the seedlings of each accession in June 10, 2015. Inoculation was continued naturally on test seedlings until the majority of the plants had reached two-expanded-leaf stage. Seedlings were maintained in the greenhouse where conditions were optimal for development of powdery mildew.
Disease scoring, area under disease progress curve (AUDPC) and relative AUDPC (rAUDPC) estimation
The first sign of infection usually appeared as small, faintly visible mildew colonies at 3 to 4 days after inoculation and disease was well developed after 7 and 11 days in 1-true-leaf and 2-true-leaf, respectively. Records on disease severity were taken on cotyledons (3, 5, and 7 days after inoculation), 1-true-leaf (7, 9, 11, and 19 days after inoculation), and 2-true-leaf (11, 13, 15, and 19 days after inoculation) stages. Disease severity was recorded on individual plant of each accession. Based on the powdery mildew symptoms developed on the host plants, a 1 to 7 scale of increasing disease severity was used (Fig. 1).
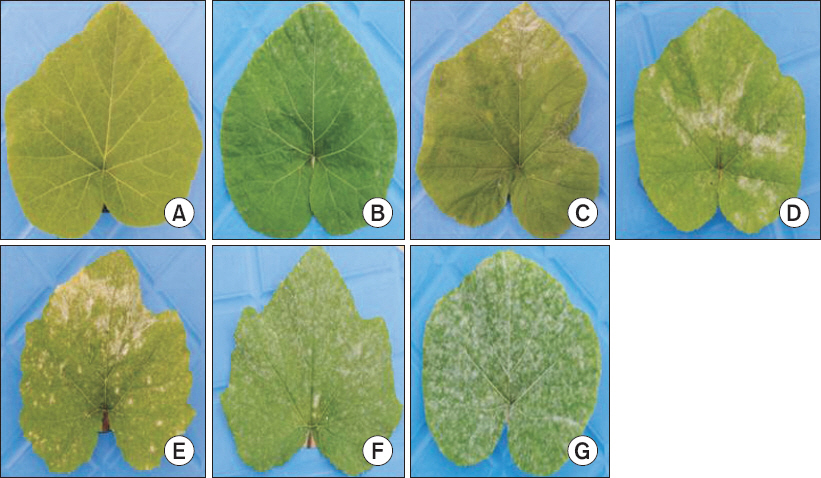
Powdery mildew scale (1–7) developed for recording disease severity in pumpkin accessions. (A) 1=no symptoms, (B) 2=10%, (C) 3=20%, (D) 4=30%, (E) 5=50%, (F) 6=80%, (G) 7=100%.
For cotyledons, 1-true- and 2-true-leaf of each accession, AUDPC was estimated using following formula;
Where, T is the time in days at ith observation and D is the estimated percentage of area of infected powdery mildew at ith observation.
Estimated AUDPCs for each interval were summed and divided by total number of days multiplied by 100. rAUDPC is an accumulated assessment of disease which lies between 0 to 1. To calculate rAUDPC, the following formula was used.
Plant growth condition and inoculum application
Seeds of 218 accessions consisting of 58, 44, 109, and 7 accessions from C. pepo, C. maxima, C. moschata, and Cucurbita sp., respectively, were sown in May 18, 2015. Seedlings were grown in 50-cell plastic plug trays filled with horticultural soil (Bio-media Co., Ltd.) for six days in glasshouse and then moved to a plastic house after the cotyledons emerged. Day temperature and relative humidity was maintained at 25°C and 70% during screening test. Inoculum was applied naturally after seven days of seedling emergence and the process of disease inoculation was same as the procedure mentioned in initial disease progress study. Disease severity was recorded on cotyledons, 1-true- and 2-true-leaf at 10 days interval and AUDPC, and rAUDPC was estimated using the method described above. AUDPC and rAUDPC were estimated in different seedling stages and accessions having rAUDPC value ranging from <0.2, <0.4, and >0.5 was classified as resistant, intermediate (moderate) resistant and susceptible, respectively.
Statistical analysis
Data were recorded on 1 to 7 scale as described previously. The rating data were first converted percentage and weighted averages were calculated for each accession. The percentage data were subjected to calculate AUDPC and rAUDPC using Microsoft Excel (version 10.0; Microsoft, Redmond, WA, USA) as the formula described previously. SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA) was used to analyze correlation coefficient among cotyledon, 1-true- and 2-true-leaf stage.
Results and Discussion
Disease severity (AUDPC) was varied in cotyledons, 1-true- and 2-true-leaf stage in the studied Cucurbita species. Looking at disease severity (AUDPC) and rAUDPC in C. pepo and C. maxima accessions, it exhibited susceptibility at cotyledonary, 1-true and even in 2-true-leaf stage but disease severity was decreased in 1-true-leaf seedling stage (Fig. 2A, Fig. 2B, respectively). However, disease severity (AUDPC) in IT 278474 of C. moschata exhibited considerable difference in different seedling stages (Fig. 2C) as compared to IT 278454. Disease severity was decreased linearly in 1-true- and 2-true-leaf than cotyledonary stage. Likewise, the accessions of C. argyrosperma exhibited decreased AUDPC and rAUDPC at 2-true-leaf stages (Fig. 2D). Relative AUDPC in IT 278463 exhibited decreasing disease severity trend in cotyledons, 1-true- and 2-true-leaf in C. pepo and similar trend was observed in the accessions of C. maxima. IT 278474 showed consistently decreased rAUDPC value indicating moderate resistant to powdery mildew at 2-true-leaf stage while all the accessions of C. argyrosperma had lower rAUDPC and it progressively decreased at different seedling stages. From the disease progress study, IT 278474, IT 278460, IT 278464, and IT 278459 displayed low rAUDPC value (<0.4) in 2-true-leaf stage indicating intermediate resistance to powdery mildew. In this study, we observed high disease severity in cotyledons in all the accessions of Cucurbita spp. but disease severity decreased linearly until 2-true-leaf stage in most of the accessions. Our results indicated that susceptibility in cotyledons was not related with susceptibility in either 1st-true and 2nd-true leaf stage under plastic house condition and Cohen and Eyal (1995) reported the similar findings in the study of C. melo. They reported that screening process undergoes until 2-true-leaf stage if cotyledons are susceptible to powdery mildew.
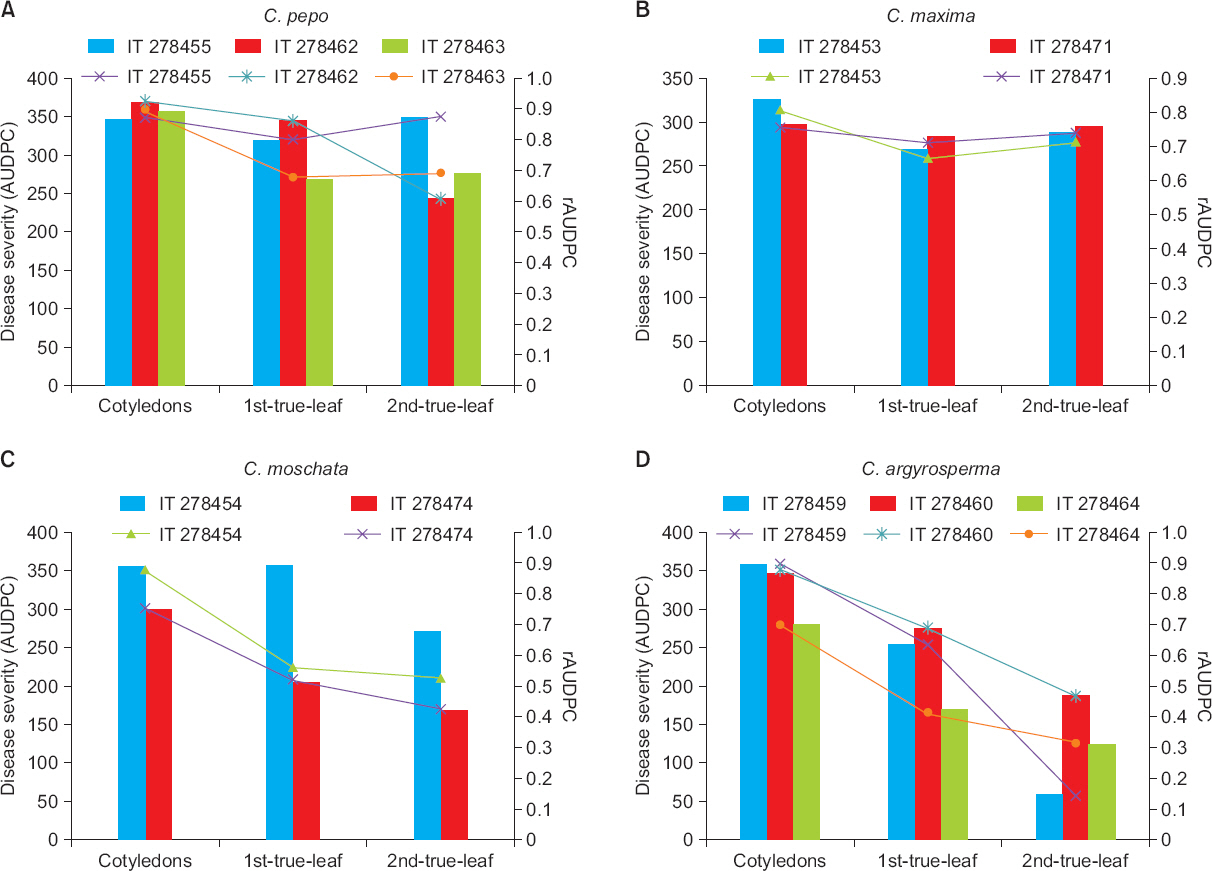
Disease severity (AUDPC) and rAUDPC of cotyledons, 1-true- and 2-true-leaf stage in the accessions of Cucurbita pepo (A), C. maxima (B), C. moschata (D), and C. argyrosperma (D). AUDPC, area under disease progress curve; rAUDPC, relative AUDPC.
Disease severity (AUDPC) and rAUDPC in cotyledonary, 1-true-leaf and 2-true-leaf stage of 218 accessions for powdery mildew in different Cucurbita species are given in Table 1. The accessions of pumpkin accessions exhibited powdery mildew susceptibility in cotyledondary stage and rAUDPC value was ranged from 0.5 to 1.0. Likewise, rAUDPC showed susceptibility to powdery mildew in 1-true-leaf stage in evaluated accessions. Of the 58 accessions of C. pepo evaluation, IT 110859 had lower AUDPC (200.0) and rAUDPC (0.2) indicating resistance to powdery mildew at 2-true-leaf stage. But IT104041 and IT 278153 were identified as moderate resistant accessions in C. pepo. Out of 44 accessions evaluated in C. maxima, five accessions (IT 108834, IT 202274, IT 277872, IT 277912, and IT 278453) showed moderate resistant (<0.4). Likewise, out of 109 accession of C. moschata tested, 12 accessions had exhibited intermediate resistance against powdery mildew and remainder accessions were observed as susceptible (>0.5). The relative AUDPC <0.2 was considered necessary for a genotype to be classed as resistant and one accession (IT 110859) had identified the resistant among Cucurbita sp. evaluated. Similarly, of the seven accession of Cucurbita sp., all were rated as susceptible. A recent study of two-hundred forty-eight accessions of pumpkin germplasm reported three accessions of C. maxima, and one accession of C. moschata were resistant (Kim et al., 2014). Similarly, in the study of Davis et al. (2001), they did not found resistant to S. fuliginea in 111 watermelon accessions. Kousik et al. (2008) evaluated 234 germplasm of Lagenaria siceraria for tolerance to powdery mildew by dusting powdery mildew spores of melon race 1 on the cotyledons but they reported none of the L. siceraria accessions were immune to powdery mildew.

Evaluation of pumpkin accessions for powdery mildew resistance in different stages of seedling development in plastic house, 2015
Our study identified one accession as resistant and 19 accessions as moderately resistant based on the evaluation of 2-true-leaf seedling stage. Our result did not show any resistant accession to powdery mildew at cotyledonary and 1-true-leaf stage exhibiting their susceptibility. In the study of Takada et al. (1974), they reported that inoculation of the first true leaf of 2-true-leaf plants was effective for screening resistant individuals. However, Cohen (1993) reported that preliminary screening can be done at cotyledonary stage using leaf disk assay. But our study employed cotyledon, 1-true- and 2-true-leaf stage to screen the pumpkin accession and disease severity decreased at 2-true-leaf stage in most of the accessions. Considering the management of powdery mildew in the field, presently, registered fungicides are available to control the powdery mildew of cucurbits. However, environmental concerns, difficulty in achieving adequate coverage, likelihood of the pathogen developing resistance to chemicals used for control and shifts in virulence of the organism mean that total reliance upon chemical means of control is insufficient (McGrath, 2001). Therefore, the best method to control powdery mildew can be the use of resistant varieties with only occasional use of chemical control if necessary (Thomas et al., 2005). Generally, breeding for resistance is based on a system of inoculation of plants with pathogen and selection of the resistant individual (Cohen and Cohen, 1986). In addition, many difficulties arise for selecting the resistance in field condition. Infection in the field is not uniform and different pathogens and various races of pathogens may also infect the plant. Powdery mildew development in the field is dependent to a great extent on environmental condition and excessive high temperature limits the development of the disease (Aust, 1986; Schnathorst, 1965). But in this study, we screened the pumpkin accessions at plastic house condition under 25°C which is optimum condition for disease development but screening for resistance to powdery mildew in greenhouse condition in three to four leaves stage might be the best method. From our study, we identified few accessions as moderate resistant and use of these moderately resistant varieties by combining with minimum spray of fungicide in greenhouse and field-grown pumpkins might be helpful to growers for the effective control of powdery mildew.
A correlation study was done in the disease severity of cotyledonary, 1-true- and 2-true-leaf stage and results are presented in Table 2. Disease severity of cotyledons inoculated with S. fuligena was moderately correlated (r=0.686**) with 1-true- and 2-true-leaf (r=402**). Similarly, disease severity between 1-true-leaf stage and 2-true-leaf stage were moderately correlated (r=0.358*). The moderate correlation indicates that the disease severity in cotyledons may not necessarily be associated with 1-true- and 2-true-leaf stage and low correlation between 1-true- and 2-true-leaf stage observed in the experiment further support this point. Previously, Pryor and Whitaker (1942) had studied about the correlation of powdery mildew development at the various stages of muskmelon plant development. They reported that resistance against Erysiphe cichoracearum in young plants in the greenhouse was not always associated with resistance in the field but we accomplished our study in plastic house condition and this suggests us to undertake our experiment at field condition for 3- and 4-leaf stages to corroborate the results. However, they reported a high degree of correlation between disease severities on cotyledons leaves and stems of the same plant in the greenhouse experiment. In the study of Ferriere and Molot (1988), they observed that muskmelon cotyledons were so susceptible to the fungus and cotyledons could not be used for screening for resistance and screening at 3-leaf stage can provide the best information on the resistance or susceptibility of genotypes to the disease.

Disease severity (AUDPC) of powdery mildew incited by Podosphaera xanthii in cotyledonary, 1-true- and 2-true-leaf stages of seedlings of pumpkin accessions
To conclude, our results clearly demonstrated that there are significant and valuable source of powdery mildew resistance in pumpkin collections. We studied the disease progress in different seedling stages of Cucurbita species. Powdery mildew affected higher in cotyledons than 1-true- and 2-true-leaf stage. Most of the accessions showed high disease severity in all seedling stages except few accessions. The initial study of disease progress established the method for screening of powdery mildew at 2-true-leaf stage and only the cotyledonary and 1-true-leaf stage cannot be used to screen against powdery mildew under plastic house condition. Out of 228 accessions screened against powdery mildew using 2-true-leaf stage, altogether two and 21 accessions from different Cucurbita species were identified as resistant and intermediate resistant, respectively and these germplasm could be used for the effective management of powdery mildew in open field and plastic house condition. Besides, screening of pumpkin accessions against powdery mildew in 3- and 4-leaf stage in field condition is recommended to confirm this finding.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgement
Financial support for the study was obtained from Research Program of Agricultural Science and Technology Development (Project No: PJ010153), National Institute of Agricultural Sciences, RDA, Republic of Korea.