body
Citrus scab diseases cause fruit blemishes that reduce the value of fruit in the fresh market. It is economically important wherever susceptible cultivars are grown for fresh fruit market in humid areas. Depending on the species or the pathotypes of scab involved, this disease may affect commercial species of citrus such as sweet orange (Citrus sinensis), lemon (C. limon), grapefruit (C. paradisi), many tangerine and mandarin lines (C. reticulata), satsuma mandarin (C. unshiu), clementine (C. clementina), their hybrids, and species used for rootstocks such as sour orange (C. aurantium), rough lemon (C. jambhiri), and cleopatra mandarin (C. reshni) (Timmer, 2000). E. fawcettii and E. australis have been recognized as the two species of scab pathogens in citrus. They cause citrus scab and sweet orange scab, respectively. E. fawcettii can be differentiated into the following six pathotypes: Florida Broad Host Range (FBHR), Florida Narrow Host Range (FNHR), Lemon, Jingeul, SRGC and Tryon’s. E. australis has two pathotypes: Sweet orange and Natsudaidai (Hyun et al., 2009).
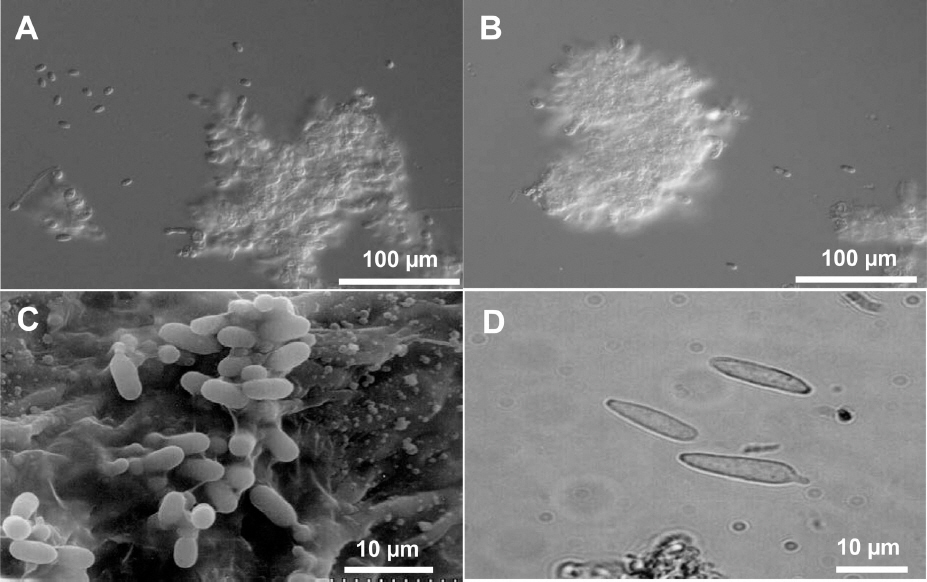
Conidia of Elsinoe spp. that cause citrus scab are hyaline, one celled, and elliptical at 3-4×4-8 mm. The conidia of all species and pathotypes of Elsinoe spp. that attack citrus are morphologically indistinguishable (Fig. 1) (Timmer, 2000). In addition to hyaline conidia, E. fawcettii produces colored and spindle-shaped conidia that can germinate and produce hyaline conidia on scab lesions, although E. australis does not produce such conidia (Fig. 1D) (Chung, 2011; Gopal et al., 2014). Because colonies of E. fawcettii and E. australis do not sporulate in artificial media including PDA, Whiteside’s method has been widely used for preparing conidia inoculum in vitro (Hyun et al., 2001; Whiteside, 1975). However, sufficient amount of conidia production for study is still a challenge, especially for some isolates or pathotypes. Therefore this study was carried out to develop a new and efficient sporulation method.
Fig. 1
Conidia of Elsinoe spp. causing citrus scab. A and B: hyaline conidia of E. fawcettii and E. australis produced in water, respectively; C: hyaline conidia on leaf lesion of E. fawcettii observed under scanning electron microscope; D: spindle-shape conidia of E. fawcettii produced on leaf lesion.
A total of 13 isolates belonging to 4 pathotypes of E. fawcettii and E. australis were selected for conidial sporulation assay (Table 1) (Hyun, et al., 2009). All isolates could grow in potato dextrose agar medium. Three weeks old colony grown on PDA of each isolate was mashed in a 90-mm diameter plastic petri dish using a spatula to obtain fungal fragments. These isolates showed variable texture and gelatinous stickiness during mashing. Based on Whiteside’s method (Whiteside, 1975), 10 ml of Fries’ medium were poured into this petri dish. The mashed mycelial fragments were vigorously stirred into the medium to separate fungal fragments. After incubating at 25°C for 2 days, the medium and any unattached hyphal fragments were decanted. Affixed mycelial fragments were flushed three times with sterile distilled water. Water was then added until micro cdonies could be barely recovered. After incubating at 25°C for approximately 12 h, the conidia were filtered with a double layer of cheesecloth and counted.
Table 1
Isolates of Elsinoe spp. used in this study (Hyun et al., 2009)
In our newly developed method, the mashed mycelial fragments were directly cultured in 50 ml sterile rain water at 25°C at 180 rpm for 24 h with shaking incubator. The culture was then filtered with a double layer of cheesecloth to eliminate culture debris and counted. Details of the two procedures used for artificial conidia production are depicted in Fig. 2. The White-side’s method needed 2 days culture in Fries’ medium, whereas the shaking method reduced the incubation period from approximately 3 days to 1 day. We compared the conidial production from the Whiteside’s method and our shaking method. The shaking method produced higher number of conidia than the Whiteside’s method for all isolates tested. Isolate SM24-4 of FBHR pathotype produced almost 34 times (12.4×107 conidia/colony) more conidia by the shaking method than the Whiteside’s method (36.4×105 conidia/colony). Jin-3 isolate of Jingeul pathotype produced least 1.8 times more conidia. Average conidial production of all isolates using the shaking method was approximately 13.1 times higher for all Elsinoe isolates. For isolates of E. fawcettii, conidia production was 14.6 times higher using the shaking method. For isolates of E. australis, conidia production was 14.2 times higher (Fig. 3).
Fig. 2
Schematic flow chart showing the preparation of conidial suspension of Elsinoe spp. using the Whiteside’s method or shaking method.
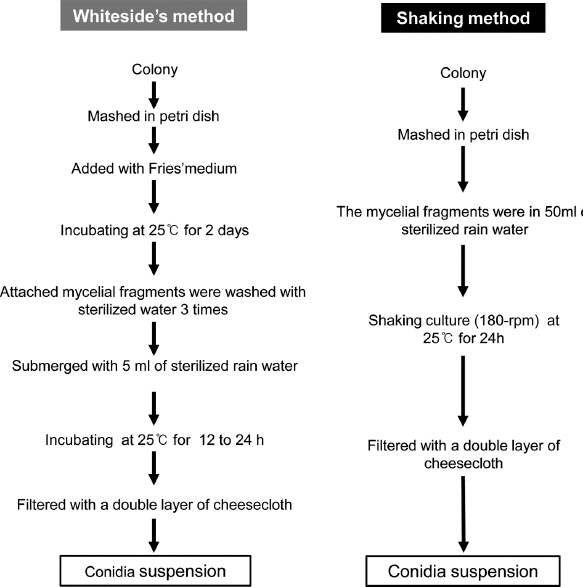
Fig. 3
Degree of conidia production from isolates of Elsinoe spp. by Whiteside's method or the shaking method. E. fawcettii : isolate SM12-1 in FNHR pathotype, Yuzu1-3, Maru-1 and SM24-4 in FBHR pathotype, Jin-1, -2, -3, -4, and -6 in Jingeul pathotype, E. australis : Na-1, Kna-2, and Kna-9 Natsudaidai pathotype.
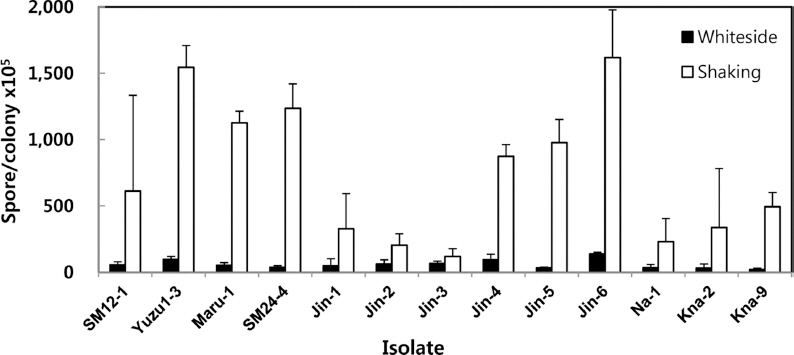
Conidia production varied among different isolates. This might be due to variations in texture, toughness, and gelatinous stickiness of isolates. These variations could potentially limit mycelial fragmentation during maceration and homogenization. Difficulty in mycelial fragmentation might interfere with the formation of conidia. Using a rotary shaker might have influenced mycelial detachment and helped the release of conidia, which increased conidia production. Therefore mycelial fragments should be cultured with shaking at 180 rpm or more for better sporulation.
It has been reported that light and physical force can influence spore release in Altarnaria (Carvalho et al., 2008) and Mycospaerella figiensis (Peraza-Echeverria et al., 2008). Leslie and Summerell (2006) have included light as another limiting factor of spore release in Fusarium spp. In addition, culture medium, alternative low-high light, and darkness can facilitate sporulation (Rodrigues et al., 2010). Limited amount of salt (CaCO3) can accelerate spore release in Altarnaria alternate, whereas high concentrations of salt can retard the release (Masangkay et al., 2000), indicating the potential role of chemicals in spore release. In this study, both methods were carried out with similar light, temperature, and final water condition. Therefore, it could be concluded that was the mycelia detachment by the shaking method that influenced the conidia production.
In here, conidia production was highly increased while the incubation time was reduced by the shaking method compared to the Whiteside’s method. Therefore, the shaking method is more effective for conidia production of citrus scab pathogen Elsinoe spp. than the Whiteside’s method.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print