Resistance of Fusarium fujikuroi Isolates to Hydrogen Peroxide and Its Application for Fungal Isolation
Article information
Abstract
The ascomycete fungus Fusarium fujikuroi causes bakanae disease in rice and this disease has been reemerging in Korea. Other fungal species including F. graminearum and Magnaporthe oryzae are often associated with F. fujikuroi, hampering pure isolation of F. fujikuroi from rice. In this study, we modified a selective medium for F. fujikuroi as supplementing both pentachloronitrobenzene and hydrogen peroxide into minimal medium. This medium efficiently suppressed the vegetative growth of F. graminearum and M. oryzae, but did not significantly reduce F. fujikuroi growth, providing an efficient tool for isolating F. fujikuroi.
body
The ascomycete fungus Fusarium fujikuroi (teleomorph Gibberella fujikuroi) causes a monocyclic bakanae disease in rice and the disease has been recently reemerging in Korea (Lee et al., 2010). Infected or infested seeds play the source of primary inoculum for disease, although ascospores produced on crop residues are reported as the primary inoculum (Leslie and Summerell, 2006; Sunder and Satyavir, 1998). Infected seeds often cause seedling to die at an early tillering stage, but usually result in typical bakanae disease symptom, the formation of elongated stems (Carter et al., 2008; Sunder and Satyavir, 1998).
To control bakanae disease, several fungicides such as prochloraz, tebuconazole, and benomyl have been widely used for seed disinfection in Korea (Kim et al., 2008; Park et al., 2009; Shin et al., 2008b). However, resistant strains against those fungicides have been occurring in field populations, resulting in dramatic increase of bakanae disease incidence in Korea (Kim et al., 2010; Shin et al., 2008a; Yang et al., 2012). Studies on population dynamics of this fungus needs for the development of suitable approaches to control this disease in rice fields. For characterization of the fungal population, it is a prerequisite to collect pure isolates from infected plants, soil, or air, but co-contamination of F. fujikuroi with other fungal species including F. graminearum and Magnaporthe oryzae in rice fields hinders pure isolation of this fungus.
For isolation of Fusarium species, media containing pentachloronitrobenzene (PCNB) such as peptone-PCNB and Komada’s medium have been broadly used (Leslie and Summerell, 2006). Recently we developed a selective medium using toxoflavin produced by the bacterial plant pathogen Burkholderia glumae (Jung et al., 2013). However, the important phytopathogenic fungus F. graminearum can grow as well as F. fujikuroi on those media even though the mycelial growth of M. oryzae is efficiently inhibited (Jung et al., 2013; Leslie and Summerell, 2006). Therefore, we need to develop a selective medium for isolating only F. fujikuroi.
Our working hypothesis was that hydrogen peroxide (H2O2) might be used for selective isolation of F. fujikuroi because Fusarium species showed different levels of resistance to toxoflavin which produces H2O2 in eukaryotic cells (Jung et al., 2013; Kim et al., 2004). We expected that it is possible to reduce the growth of certain fungal species as supplementing both H2O2 and PCNB into a medium. As a result, this study showed that the vegetative growth of F. graminearum and M. oryzae was completely inhibited but F. fujikuroi can grow on the medium containing both PCNB and H2O2. This result provides an efficient tool for F. fujikuroi isolation as inhibiting the growth of other pathogens including F. graminearum and M. oryzae, and can be applicable for characterizing population dynamics of F. fujikuroi in rice fields.
For this study, 77 F. fujikuroi strains were isolated from rice seeds collected from four provinces, Gangwon, Gyeonggi, Chungnam, and Chungbuk in 2013. Each fungal strain was identified based on morphological characteristics including pigmentation, hyphal growth, and spores on potato dextrose agar (PDA), carnation leaf agar, and carrot agar (Leslie and Summerell, 2006). The morphological characteristics was compared with the phylogenetic characteristics obtained as sequencing partial translation elongation factor 1-alpha (TEF) DNA sequences amplified with the PCR primers, ef1 (forward primer; 5’-ATGGGTAAGGA(A/G)GACAAGAC-3’) and ef2 (reverse primer; 5’-GGA(G/A)GTACCAGT(G/C)ATCATGTT-3’) (O’Donnell et al., 1998). The TEF sequences were compared to the Fusarium-ID with standard nucleotide BLAST (http://isolate.fusariumdb.org/). F. graminearum strains GJ90 and IS40 previously isolated in Korea (Jung et al., 2013), and GZ3639 and SCK04 strains provided from Center for Fungal Pathogenesis (Seoul, Korea) were used for reference strains of F. graminearum. M. oryzae strains were provided from Center for Fungal Genetics Resources (Seoul, Korea).
To prepare PCNB-H2O2 medium, 0.75 g of PCNB (Sigma-Aldrich) dissolved in 75 ml of ethanol was combined with with 1 l of minimal medium (MM) agar (Leslie and Summerell, 2006). The medium was autoclaved and allowed to cool to 50°C before adding 0 to 5 mM H2O2 (Sigama-Aldrich). After inoculating each fungal strain onto the medium, the plates were incubated at 25°C in dark condition and mycelial growth was measured every 24 h until 7 days after inoculation. The experiments were repeated three times with three replicates and Tukey test using SPSS 12.0 software (SPSS Inc., Chicago, USA) was performed to examine the significant differences (P<0.05) of mycelia growth among the mean values of the samples.
On MM supplemented with H2O2, F. graminearum and F. fujikuroi strains were well grown, but F. graminearum produced less carmine red pigment as supplementing more H2O2 while pigmentation of F. fujikuroi was not affected by the concentration of H2O2 tested in this study. On the other hand, M. oryzae strains were more sensitive to H2O2 where its growth was reduced as supplementing H2O2 and completely inhibited at 5 mM H2O2 (Fig. 1). This result showed that fungal pathogens isolated from rice have different sensitivity against H2O2 and F. fujikuroi strains are more tolerant to H2O2 than F. graminearum and M. oryzae strains.

Vegetative growth of fungal strains on minimal medium (MM) supplemented with various H2O2 concentration (1, 3, and 5 mM). FG, Fusarium graminearum; CF242 and CF375, F. fujikuroi strain CF242 and CF375; MO, Magnaporthe oryzae. Photograph was taken five days after inoculation.
On peptone-PCNB agar (PPA) (Leslie and Summerell, 2006), all F. graminearum strains were well grown as much as peptone agar (PA), but the growth of F. fujikuroi and M. oryzae strains were slightly reduced compared to that on PA (Table 1). Fungal strains tested in this study did not form distinctive colonies on PPA and we tested the fungal colony formation on different media containing PCNB. When F. graminearum and F. fujikuroi species were incubated on MM supplemented with PCNB, they produced similar colony pigmentation patterns as shown on MM without PCNB even though their mycelial growth was decreased responding to the addition of PCNB (Table 1). The growth of M. oryzae was significantly inhibited on MM containing PCNB even though it grew on PPA. This result showed that F. graminearum can be efficiently selected on MM-PCNB rather than PPA because the fungal strains produce carmine red pigment and the growth of other fungal competitors, such as F. fujikuroi and M. oryzae, on rice are inhibited.
We further tested the availability of H2O2 for isolating F. fujikuroi on MM containing PCNB. As increasing H2O2 concentration, the mycelia growth of F. graminearum significantly slowed down and it was completely inhibited at the addition of 5 mM H2O2. In the case of F. fujikuroi, its growth was not inhibited at the presence of H2O2. The growth of M. oryzae was completely inhibited at 3 mM H2O2 (Table 1). This result showed that the addition of H2O2 into MM-PCNB efficiently can inhibit the growth of F. graminearum and M. oryzae except for F. fujikuroi, providing an efficient tool for isolating only F. fujikuroi strains. To determine whether F. fujikuroi field isolates from different regions in Korea are resistant to MM-PCNB-H2O2 medium we developed in this study, we tested the efficacy of the medium targeting for 77 F. fujikuroi strains isolated from different rice fields in Korea. Sixty six out of the strains grew but rest 11 strains significantly slowed down on the medium.
To test whether the resistance of F. fujikuroi against fungicides affects the resistance to MM-PCNB-H2O2 medium, all of the 77 field strains were inoculated on MM containing prochloraz (23% EC; Kyung Nong, Seoul, Korea), tebuconazole (25% ME; KC Life Science, Seoul, Korea), or benomyl+thiram (20%+20% WP; Hankook Samgong, Seoul, Korea). The final concentration of fungicide in the medium was 0.3 μg/ml for prochloraz, 5 μg/ml for tebuconazole, and 5 μg/ml for benomyl+thiram. The mycelial growth of each strain was measured 4 days after inoculation.
Out of 77 F. fujikuroi strains, 49, 23, and 26 strains were resistant to prochloraz, tebuconazole, and benomyl+thiram, respectively (Fig. 2). The data showed that the resistance against either prochloraz or tebuconazole was not significantly associated with PCNB-H2O2 resistance. However, the number of resistant strains against benomyl+thiram might be associated with PCNB-H2O2 resistance and especially the number of resistant strains against both prochloraz and benomyl+thiram was highly skewed toward non-resistance against PCNB-H2O2 resistance. This result suggested that the application of new fungicide into fields significantly affects the population dynamics of this fungus and the resistance pattern might be one of important characteristics to understand population structure of this fungus.
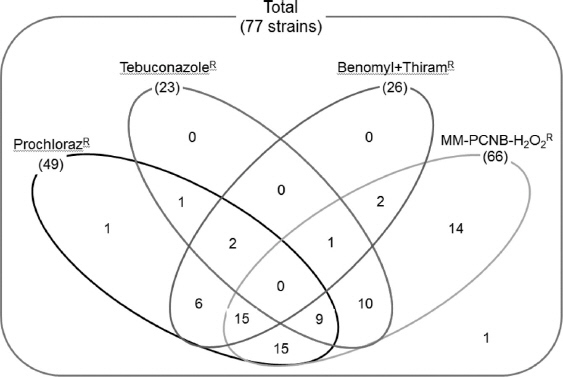
Resistance of Fusarium fujikuroi strains to different fungicides. The numbers in the parentheses indicate the number of resistant strains to the fungicide.
Although media containing PCNB have been widely used for isolation of strains belonging to the genus Fusarium, a selective medium for F. fujikuroi isolation has not been developed. In this study, we modified the MM as supplementing both PCNB and H2O2 to keep the vegetative growth of F. fujikuroi but to inhibit that of F. graminearum and M. oryzae. Approximately 85% of F. fujikuroi strains tested in this study were strongly resistant on the medium developed in this study but 15% were sensitive to the medium, suggesting that this medium might not be used for all field population. However, this medium can be applicable for population study to chase population dynamics related to fungicide resistance and also useful for field study examined with limited number of F. fujikuroi strains.
Acknowledgement
This work was supported by Rural Development Administration (PJ0098912015).
