Introduction
The storage of seeds as grain, other staple food and feed free of fungal contamination is of importance to consumers (Agarwal and Sinclair, 1996). Storage fungi of seeds comprising mainly species of Aspergillus and Penicillium normally do not play a role in disease development in the field but play a major role in seed deterioration in storage (Agarwal and Sinclair, 1996). Decrease in germinability, discoloration and shrinkage of the grains, heat damage by fungal respiration, and chemical breakdown of nutrients can be caused by interactions which occurred between fungi and the stored seeds. Moreover, they may produce mycotoxins that are injurious to human and animals.
Several chemicals have been used for disinfection of stored seeds, fruits and tubers to prevent insect damages which play an important role in making seeds vulnerable to fungal infection and distribution of fungal inocula. Phosphine, methyl bromide, ethylene and propylene oxides have been often used as fumigants to control insects (Sittisuang and Nakakita, 1985). Acetaldehyde, propanol, and butanal were tested on cherry fruit to control P. expansum conidial germination (Matthesis and Roberts, 1993). Mercuric chloride which had been used for surface sterilization of seeds is seldom used today as it is dangerous to use, and is difficult to dispose. Gamma irradiation is used to sterilize seeds without decreasing seed viability (Cuero et al., 1986), but seed germination, seed membrane permeability and nutritional value were significantly altered in radiation-exposed wheat seed (Maity et al., 2009). It is necessary to develop an effective seed disinfectant without toxicity to human and animals, effect on seed germinability and risk on abnormality of seedlings.
Chlorine has been used for surface sterilization of seeds (Sauer and Burroughs, 1986). It is easily available and very cheap, however, it has disadvantages including extensive corrosion of metal equipment, sensitivity to organic load, and the formation of chlorinated byproducts (Hikada et al., 1992). For these reasons, needs for new methods to disinfect storage fungi in seeds have been increasing. Chlorine dioxide (ClO2) is less toxic than common chlorine, produces no byproduct, and has no particular odor or taste (Dietrich et al., 1992; Wondergem and van Dijk-Looijaard, 1991). Aqueous ClO2 was reported as a highly efficient disinfectant against fungi including Alternaria sp. and Penicillium sp. (Errampalli et al., 2006), and Botrytis cinerea, P. expansum and Rhizopus stolonifer (Zoffoli et al., 2005). Gaseous ClO2 was effective for controlling bacterial postharvest decays of apple (Lee et al., 2006), tomato (Mahovic et al., 2007) fruits. Trinetta et al. (2011) found that was effective for inactivation of Salmonella enterica and Escherichia coli O157:H7 on preinoculated seeds of tomato, lettuce and cantaloupe. Disinfection of seeds with gaseous ClO2 may be useful for re-storage or transportation because it is a dry system.
The aims of this study were to reveal whether gaseous ClO2 can inhibit growth of Penicillium sp. in wheat seeds and which condition is the best for disinfection of them. The effectiveness of gaseous ClO2 was evaluated by investigating the incidence of Penicillium sp. in wheat seeds treated with various concentrations of ClO2, relative humidity in treatment chamber and physical conditions of the seed. The toxicity of gaseous ClO2 to the seed was evaluated by seed germination test.
Materials and Methods
Seed material
Seeds of wheat, Triticum aestivum L. cv. Olgeurumil, were harvested at Suwon, Republic of Korea and stored in a mesh bag at 4-10┬░C for 15 years. Mean germination rate was 42.9% as determined by between-paper method.
Incidence of Penicillium sp. in wheat seed.
Fifty seeds were placed equidistantly on KomadaŌĆÖs medium in 90-mm Petri dishes (25 seeds per dish) and incubated at 25┬░C in the darkness for 7 days. The incidence of Penicillium sp. in seed was expressed as a percentage of the number of seeds with fungal growth to the number of seeds tested.
Seed germination.
One-hundred seeds were tested for germination using the between-paper method. Seeds were placed equidistantly between two layers of germination paper (Anchor paper Co., MN, USA) moistened with distilled water, after which the papers were loosely rolled up and placed in a plastic bag in an upright position to reduce surface evaporation. After incubation at 20┬░C in the darkness, the number of seeds that germinated normally after 4 and 8 days was determined and germination rate was expressed as a percentage of the number of normal seedlings to the number of seeds tested.
Seed moisture content.
One-hundred seeds were ground and dried at 130┬░C for 2 hours in aluminum container, then they were allowed to cool in a desiccator for 40 min. The weights of empty containers (W1), the weights of seeds and containers before drying (W2) and the weights of seeds and containers after drying (W3) were measured to four decimal places. The seed moisture content (SMC) was calculated using the formula, (W2 - W3)/(W2 - W1) ├Ś 100, and expressed as percentage to one decimal place. SMC of 30% was accomplished by soaking dry sees in distilled water at 25┬░C for 24 hours.
ClO2 treatment.
Gaseous ClO2 was generated by UV irradiation of Bactericide┬« gel (Oxy Therapy, Rep. of Korea) containing 2% (w/w) ClO2. Within a treatment chamber, the ClO2 concentration was measured by means of a portable detector (PortaSensII, Applied Technology Inc., USA). The relative humidity (RH) of the chamber was adjusted to 30, 50, 70 and 80% using a hand-made RH controller. Temperature within treatment chamber was kept around 23┬░C. To enable contact of seeds with ClO2 during treatment, three hundred seeds were spread in a single layer without overlapping in a polypropylen mesh bag and placed on a tube rack. For treatment of Penicillium sp., fragment of 10-day-old fungal cultures grown on wheat seeds was suspended by sterile distilled water in an Eppendorf tube. The resulting suspension was manually counted with a haemocytometer and diluted in sterile water to 1 ├Ś 103 conidia/ml. Two hundred microliters of the diluted suspension was spread on potato dextrose agar (PDA) medium in 90 mmdiameter Petri dish and then exposed to gaseous ClO2 with half of the lid open.
Component plating test.
Twenty-five seeds were used for detecting the Penicillium sp. associated with different components of wheat seeds. Seeds were soaked in sterile distilled water at room temperature for 24 hours. Seed components were separated into endosperm with bran and embryo using a sterile scalpel under a stereomicroscope. Each component was placed on PDA medium and incubated at 25┬░C for 7 days under alternating 12-h near UV and 12-h dark.
Statistical analysis.
Each experiment was conducted three times except the measurement of SMC. An arc sine transformation was performed for percentage data. Analysis of variance and DuncanŌĆÖs new multiple range tests with the package ŌĆśagricolaeŌĆÖ (Mendiburu, 2014) was conducted using R version 3.1.0 (R Core Team, 2014). Significant differences were determined at the 95% confidence level (p Ōēż 0.05).
Results and Discussion
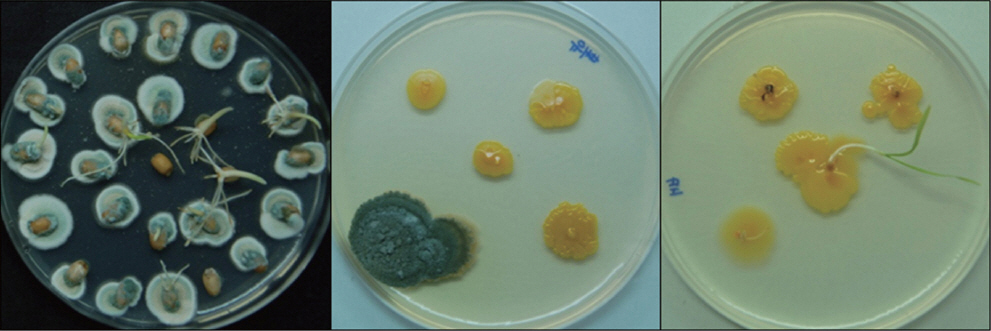
Wheat seeds used in this study appeared to be relatively healthy without any shrinkage, discoloration and disease symptoms. There was, however, breakage of pericarp throughout the seeds (data not shown). Damage caused by breakage of the protective outer seed layers can provide sites for fungal infection (Agarwal and Sinclair, 1996). Wheat seeds were infected with Penicillium sp. at mean infection rate of 83% as determined by incubation on KomadaŌĆÖs medium. Penicillium sp. was detected from endosperm with bran but not from embryo indicating that Penicillium sp. mainly existed near the seed surface (Fig. 1).
Fig.┬Ā1
Detection of Penicillium sp. from whole wheat seed (left), endosperm and bran (middle) and embryo (right). Each component was placed on PDA medium and incubated at 25┬░C for 7 days under alternating 12-h near UV and 12-h dark.
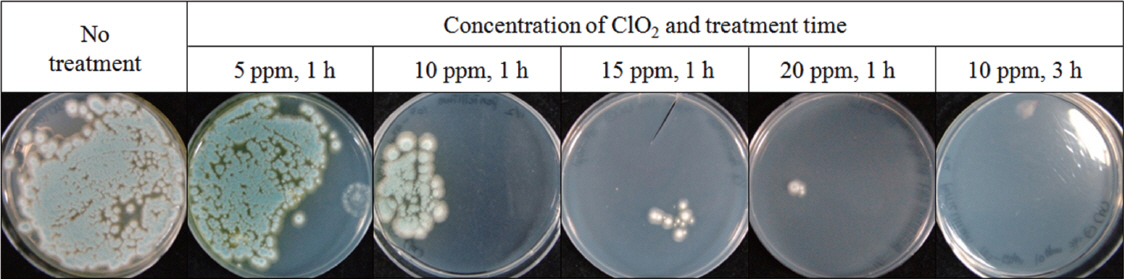
ClO2 has received attention as a disinfectant for post-harvest fungal pathogens because of its stable efficacy and oxidizing power (Beuchat, 1998). Gaseous ClO2 is expected to be more advantageous to disinfection of dry seeds because aqueous ClO2 would increase the SMC, which may promote fungal growth and may reduce seed viability during storage. Gaseous ClO2 inhibited growth of Penicillium sp. isolated from wheat seeds on PDA as the ClO2 concentration increased from 5 to 20 ╬╝g/ml (Fig. 2). When treatment time was extended from 1 to 3 h, growth of Penicillium sp. was completely suppressed even at 10 ╬╝g/ml. Gaseous ClO2 treatment was found to effectively suppress growth of Penicillium sp. in vitro. Extending treatment time was more effective for inhibition than increasing ClO2 concentration.
Fig.┬Ā2
Growth inhibition of Penicillium sp. by ClO2 treatment. Two-hundred conidia were spread on PDA medium in 90 mm-diameter Petri dishes and then exposed to each concentration of ClO2 with the half of the lid open. The treated conidia were incubated at 25┬░C for 7 days.
Moisture content around seeds during ClO2 treatment was controlled by adjusting RH in the treatment chamber. Dry seeds were exposed to 20 ╬╝g/ml for 10 h at 30, 50, 70 and 80% RH (Table 1). There was no significant reduction in the incidence of Penicillium sp. at 30% RH. However, reduction in the incidence of Penicillium sp. to 27.7% was observed at 50% RH, further reduction to 3.5% and 0.2% was accomplished at 70% and 80% RH, respectively. Seed germination was not affected by ClO2 treatment at all the RH conditions. A high RH around the seed surface increased the effectiveness of ClO2. Base on the result, a RH higher than 70% is recommended. Moisture content in the dry seeds was increased to 30.0% by soaking in the water. The incidence of Penicillium sp. in dry seeds with 9.7% SMC did not show any reduction when treated at 5 and 10 ╬╝g/ml at 50% RH, however, it tended to decrease as ClO2 concentration increased to 20 ╬╝g/ml (Table 2). Water-soaked seeds showed a drastic reduction in the incidence of Penicillium sp. when treated at more than 10 ╬╝g/ml. The incidences of Penicillium sp. were 3.3, 1.8 and 1.2% at 10, 15 and 20 ╬╝g/ml, respectively. It should be noted that the incidence of Penicillim sp. was reduced to 38.8% in water-soaked seed even without ClO2 treatment, which was believed to be a result of secession of bran containing conidia or mycelia of Penicillim sp. during 24-h soaking. Seed germination was not affected by ClO2 treatment, regardless of concentration. Increasing water content around or inside of the seeds during ClO2 treatment strongly increased the effectiveness of disinfection. It is possible that water replaced the air between endosperm and bran of the seeds, which promoted diffusion of ClO2, and activated sleeping pathogen, causing the pathogen to become more susceptible to ClO2 (Agarwal and Sinclair, 1996). Above all, water might serve a medium for ClO2 action in the seed, where the pathogens existed.
Table┬Ā1
Incidence of Penicillium sp. and seed germination of wheat seeds after ClO2 treatment at various conditions of relative humidity (RH)
| No treatment | RH during ClO2 treatment (%)z | ||||
|---|---|---|---|---|---|
|
|
|||||
| 30 | 50 | 70 | 80 | ||
| Incidence of Penicillium sp. (%)y | 47.0 a | 47.5 a | 27.7 b | 3.5 c | 0.2 c |
| Seed germination (%)y | 42.2 a | 43.8 a | 42.0 a | 42.8 a | 43.7 a |
Table┬Ā2
Incidence of Penicillium sp. and seed germination in dry and soaked wheat seeds after ClO2 treatment
| Seedy | Concentration of ClO2 (╬╝g/ml)z | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| 0 | 5 | 10 | 15 | 20 | ||
| Incidence of Penicillium sp. (%)x | Dry | 47.5 a | 42.5 b | 44.2 b | 34.8 d | 24.7 e |
| Soaked | 38.8 c | 21.0 f | 3.3 g | 1.8 g | 1.2 g | |
|
|
||||||
| Seed germination (%)x | Dry | 43.7 a | 41.0 a | 43.0 a | 42.3 a | 42.8 a |
| Soaked | 43.5 a | 41.5 a | 41.3 a | 42.2 a | 42.5 a | |
Wheat is the main cereal crop as it provides 20% of all calories consumed by people worldwide and also makes a significant contribution to animal feed. Contamination of wheat seed with storage fungi including Penicillium sp. may cause problems. Production of mycotoxins and loss of nutritive value by storage fungi are harmful or disadvantage for human and animal feed consumption. Deterioration of seeds by storage fungi becomes problematic for cultivation because deteriorated seeds may have the potential for low germinability and production of abnormal seedlings. The FDA has permitted the use of ClO2 for disinfecting meat, poultry, fruit and vegetables for human consumption in an amount not to exceed 5 ╬╝g/ml residual ClO2 for uncut fruits and vegetables. Our results indicated that there was a statistically significant effect of gaseous ClO2 on disinfection of Penicillium sp. in wheat seeds at sites where moisture existed (30% SMC or > 70% RH). Gaseous ClO2 treatment is expected to be effective and safe method for disinfection of Penicillim infected wheat seeds.
Further investigations should be conducted to ascertain the best conditions for complete disinfection of Penicillium sp. in wheat seeds. We need to ascertain that ClO2 treatment affect seed germination with highly germinable seed material. Once these problems are addressed, gaseous ClO2 treatment can be applied to disinfection of Penicillium-infected wheat seed as an effective but less toxic method.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print