First Report of Soft Rot Induced by Dickeya dadantii on Euphorbia hypogaea in Korea
Article information
Abstract
In a survey conducted in March 2023, Euphorbia hypogaea plants cultivated within greenhouses in Yongin, Korea exhibited water-soaked areas near the stem base, close to the soil. Subsequent isolation from diseased E. hypogaea led to the identification of a bacterial strain, designated as CBNUMPBL-103. The isolate was identified as Dickeya dadantii through sequencing of the 16s rRNA and phylogenetic analysis. The pathogenicity of the isolate was confirmed by inoculating it into healthy E. hypogaea, resulting in the manifestation of similar symptoms observed during the survey. The re-isolated strain recovered from inoculated plants showed a similar morphology with the inoculated strain. This is the first documentation of D. dadantii causing soft rot of E. hypogaea in Korea.
Ornamental plants hold a great cultural significance often used in ceremonies and given as gifts during celebrations. They also contribute to the aesthetic appeal of homes, hotels, restaurants and wedding halls. Succulents, known for their ability to withstand environmental stress are particularly popular as potted ornamental plants. In Korea, they stand out as the top choice for floral exports, generating approximately $4.9 million in revenue across more than 20 countries (Korea Agro-Fisheries & Food Trade Corporation, 2022). Euphorbia, a member of the spurge family (Euphorbiaceae), originates from South Africa, where it predominantly thrives in desert environments (Moller and Becker, 2019). This plant gained popularity as ornamental choice due to its striking resemblance to pineapple and its low-maintenance requirements, needing minimal watering. Despite the popularity of succulents, they face challenges especially from insect pests and diseases that can reduce their productivity. Soft rot has been reported to affect ornamental crops in Korea and various other countries, with the identified causal pathogens being Pectobacterium and Dickeya, formerly classified in the genus Erwinia (Baghaee-Ravari and Gerayeli, 2015; Choi and Lee, 2000; Hahm et al., 2003; Joko et al., 2014; Kim et al., 2007).
Dickeya spp. formerly referred to as Erwinia chrysanthemi, is notorious for causing significant diseases in potato and horticultural crops (Carstens et al., 2019). A significant species from this genus is D. dadantii, recognized as an opportunistic pathogen primarily infecting storage organs and fleshy tissues. Additionally, its association with systemic infections and vascular tissues further underscores its broad range of detrimental effects within affected plants (Glasner et al., 2011). Instances of Dickeya-induced potato blackleg have been reported in Japan (Fujimoto et al., 2018), Thailand (Kumvinit and Akarapisan, 2019), and the United States, where an outbreak was linked to Dickeya spp. (Curland et al., 2021). Additionally, in South Korea, cases of Chinese cabbage and potato blackleg caused by Dickeya were also reported (Seo et al., 2004).
We conducted a survey of ornamental crop diseases in Yongin, Gyonngi-do province, Korea, and our observations revealed a concerning disease affecting E. hypogaea plants cultivated in greenhouses. These plants displayed rotting symptoms near the soil line around the base of their stems. Affected plants experienced a rapid decline, withering, and ultimately fell off within a few days of being infected. Detailed examination of the affected plants revealed symptoms consistent with soft rot, characterized by a watery decay that progressively led to the affected region losing its firmness, ultimately resulting in the collapse of the entire plant (Fig. 1A).

(A) Disease symptoms observed on Euphorbia hypogaea during survey. The affected plants exhibited rotting on the succulent stem and branches, leading to their collapse. (B) In the laboratory, bacteria were isolated from the diseased E. hypogaea. Plate shows colonies of CBNUMPBL-103 on Tryptic Soy Agar medium (Difco, Ponte de claix, France).
In order to isolate the causal pathogen, symptomatic plants were first cleaned with water to remove adhering soil particles. Diseased tissue was cut and gently crushed in sterile distilled water (SDW) and 20 µl of tissue extract streaked onto Tryptic Soy Agar (TSA) medium (Difco, Ponte de claix, France). The plates were incubated at 28°C for 48 h. The colonies observed were transferred to freshly prepared TSA to obtain pure culture. The isolate obtained was designated CBNUMPBL-103. The colonies on TSA were round, flat, white with irregular margins 48 h after inoculation (Fig. 1B). Subsequently, the colonies turn to light yellow, merging together to create larger colonies 7 days after inoculation.
Pathogenicity test of the isolate was conducted on potted E. hypogaea under greenhouse conditions. The plants were inoculated by drenching with 1×109 cfu/ml (OD600=0.1) inoculum suspension of the isolates on the soil in contact with the stem. SDW was used as negative control. Symptoms only appeared on plants inoculated with isolate CBNUMPBL-103 with all the inoculated plants showing symptoms 24 h after inoculation while control had no disease symptoms. The symptoms were characteristic of soft rot which were slimy rot observed at the base of the plant. The leaves also showed black discoloration and severe wilting (Fig. 2A). The disease scoring on the scale of 0 to 4 (0, no disease symptom; 4, severe rot) showed a dramatic increase of symptoms reaching the maximum score 4 days after inoculation (Fig. 2B). The bacteria responsible for the disease was isolated again, and it exhibited colony characteristics consistent with CBNUMP-BL-103.

Pathogenicity of Dickeya dadantii on Euphorbia hypogaea. (A) Comparison of symptoms between the plant inoculated with CBNUMPBL-103 strain and plant inoculated with sterile distilled water (SDW). The inoculated plant exhibited rotten leaves that turned black and a rotten stem (left), while the healthy plant inoculated with SDW remained unaffected (right). (B) Disease progression on inoculated plant showing steady increase in disease index from inoculation to 4 days after inoculation.
For molecular identification, the DNA of CBNUMPBL-103 was isolated using Exgene TM Cell SV DNA isolation kit (GeneAll, Seoul, Korea) according to the manufacturer's instructions. Polymerase chain reaction (PCR) was used to amplify the 16S rDNA in a reaction volume of 25 µl containing 1 µl of 20 ng total DNA; 1 µl each of the 342F primer (5’-CTACGGGGGGCAGCAG-3’) and 806R primer (5’-GGACTACCGGGGTATCT-3’) each at the concentration of 10 pmole/µl; 2 µl of 10 mM dNTP; 2.5 µl of 5x TB buffer; and 1 µl of Taq DNA polymerase. The PCR reaction conditions were as follows 95°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec and extension at 72°C for 1 min, a final extension at 72°C for 10 min. The PCR products were purified and sequenced by Macrogen, Inc. (Seoul, Korea). BLAST analysis of the sequence at National Center for Biotechnology Information (NCBI) database showed that the isolate CBNUMPBL-103 had 99% similarity with D. dadantii. The sequence of the isolate was deposited in the GenBank with accession number OR965011. The identity of the isolate was confirmed by phylogenetic analysis using reference sequences of Dickeya spp. obtained from the NCBI GenBank. Pectobacterium carotovorum was used as an outgroup. The sequences of 16S rDNA were aligned in MEGA11: molecular evolutionary genetics analysis version 11 for Windows (Tamura et al., 2021). The aligned sequences were used to construct a phylogenetic tree (Fig. 3) using the Maximum likelihood method.
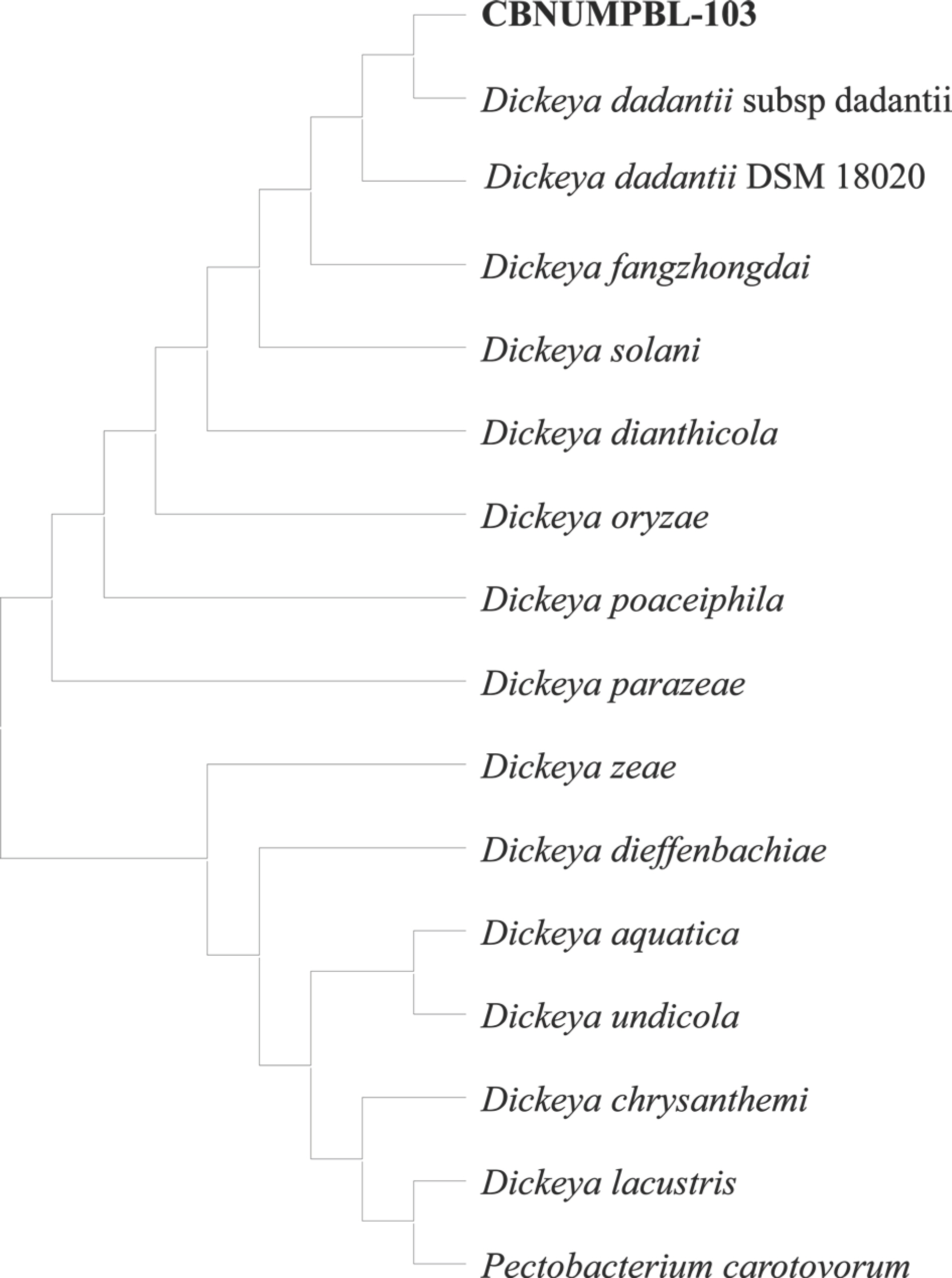
Phylogenetic tree generated through the maximum likelihood method, showing the clustering of CBNUMPBL-103 isolate alongside reference isolates of Dickeya dadantii sourced from the National Center for Biotechnology Information database.
This study has demonstrated that D. dadantii is a causative agent of disease in succulent plants in Korea. Consequently, it is crucial to undertake a thorough survey to assess the prevalence of soft rot induced by D. dadantii, particularly in succulent ornamental crops. Such a survey would provide valuable information for making informed decisions aimed at minimizing losses of succulent crops in the rapidly growing floral industry in Korea.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The authors acknowledge the support of Kim, M. K and Kim, H. T during disease assessment and sample collection.
