Aime, M. C. 2006. Toward resolving family-level relationships in rust fungi (Uredinales).
Mycoscience 47: 112-122.

Aime, M. C., Bell, C. D. and Wilson, A. W. 2018. Deconstructing the evolutionary complexity between rust fungi (
Pucciniales) and their plant hosts.
Stud. Mycol. 89: 143-152.



Benjamin, M. A. Z., Ng, S. Y., Saikim, F. H. and Rusdi, N. A. 2021. Ethnobotany and traditional knowledge of bamboos (Poaceae: Bambusoideae) in Asia and their applications in the complementary and alternative medicine: a review.
Pharmacogn. J. 13: 1751-1762.

Choi, M. H., Seo, Y. J. and Shin, H. J. 2017. Application of domestic bamboo stems mainly for inner beauty product development: a review.
KSBB J. 32: 1-8. (In Korean)

Cummins, L. M. and Kimura, E. T. 1971. Safety evaluation of selenium sulfide antidandruff shampoos.
Toxicol. Appl. Pharmacol. 20: 89-96.


Dey, S., Biswas, S., Kundu, A., Pal, A. and Das, M. 2023. Current understanding on major bamboo diseases, pathogenicity, and resistance genes. Genetics, Genomics and Breeding of Bamboos,. M. Das, L. Ma, A. Pal and C. Kole 256-278. CRC Press; Boca Raton, FL, USA.

Gardner, D. E. and Hodges Jr, C. S. 1989. The rust fungi (Uredinales) of Hawaii. Pac. Sci. 43: 41-56.
Hiratsuka, N. 1992. The rust flora of Japan. Tsukuba Shuppankai, Tsukuba, Japan.
Huang, L., He, J., Tian, C. M. and Li, D. W. 2023. Bambusicolous fungi, diseases, and insect pests of bamboo.
For. Sci. 3: 415-440.

Katumoto, K. 1968. Notes on the bambusicolous rust fungi with special reference to Japanese species. Bull. Fac. Agric. Yamaguchi Univ. 19: 1135-1158.
Kumar, S., Stecher, G., Li, M., Knyaz, C. and Tamura, K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms.
Mol. Biol. Evol. 35: 1547-1549.



Lee, T. S. 2001. Pucciniaceae of Korea. National Institute of Agricultural Science and Technology. Suwon, Korea.
Lee, Y. C., Lee, M. S. and Jang, S. J. 2016. Jukyeom as the source of trace elements to the human body: an analysis using Insan Jukyeom.
J. Food Sci. Nutr. 2: 011. (In Korean)

Magnus, P. 1899. Über die bei verwandten Arten auftretenden Modifikationen der Charaktere von Uredineengattungen. Ber. Dtsch. Bot. Ges. 17: 178-184.
Nelson, S. and Goo, M. 2011. Kweilingia rust of bamboo in Hawaii. University of Hawaii, Honolulu, HI, USA. pp. 5 pp.
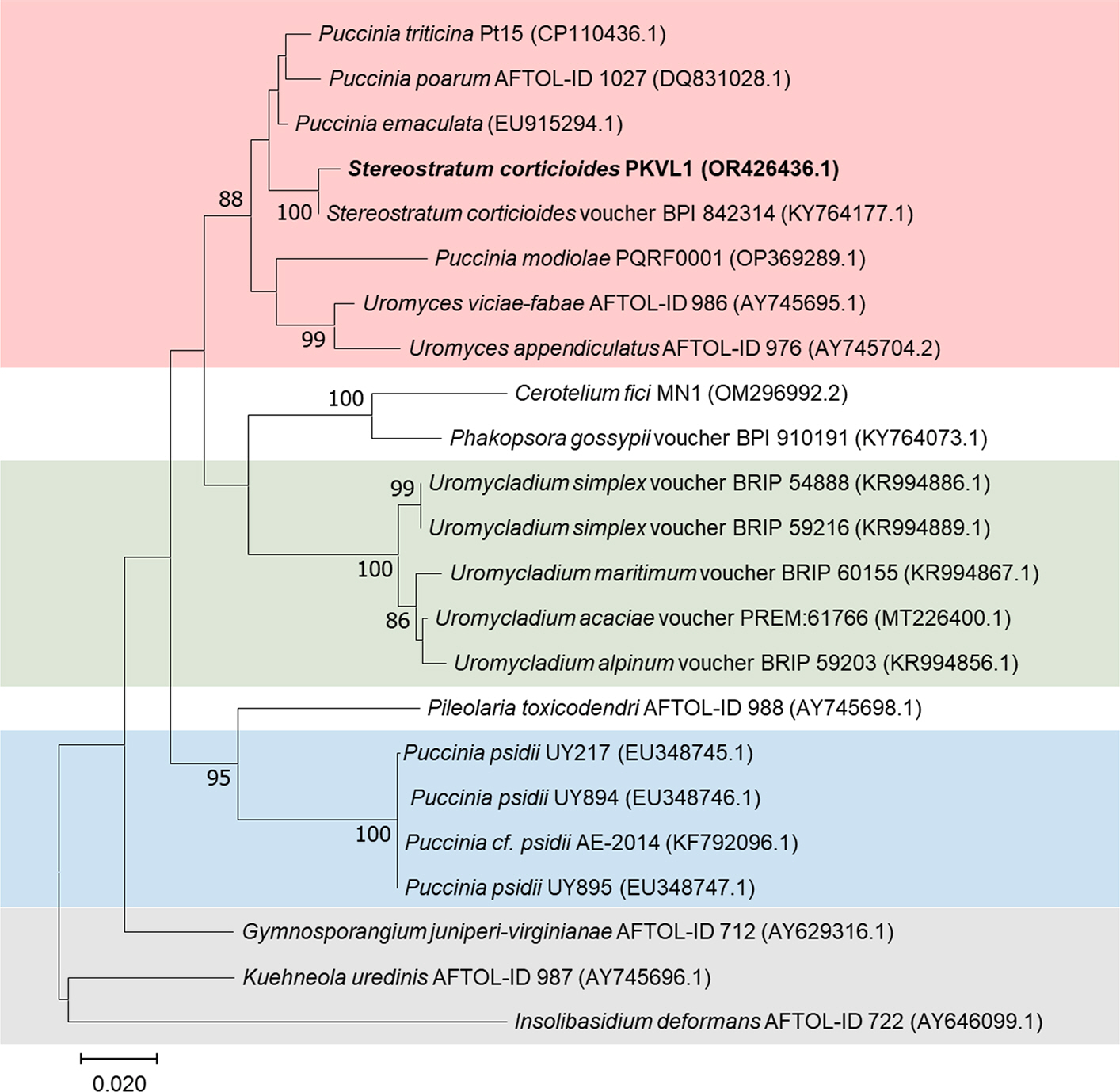
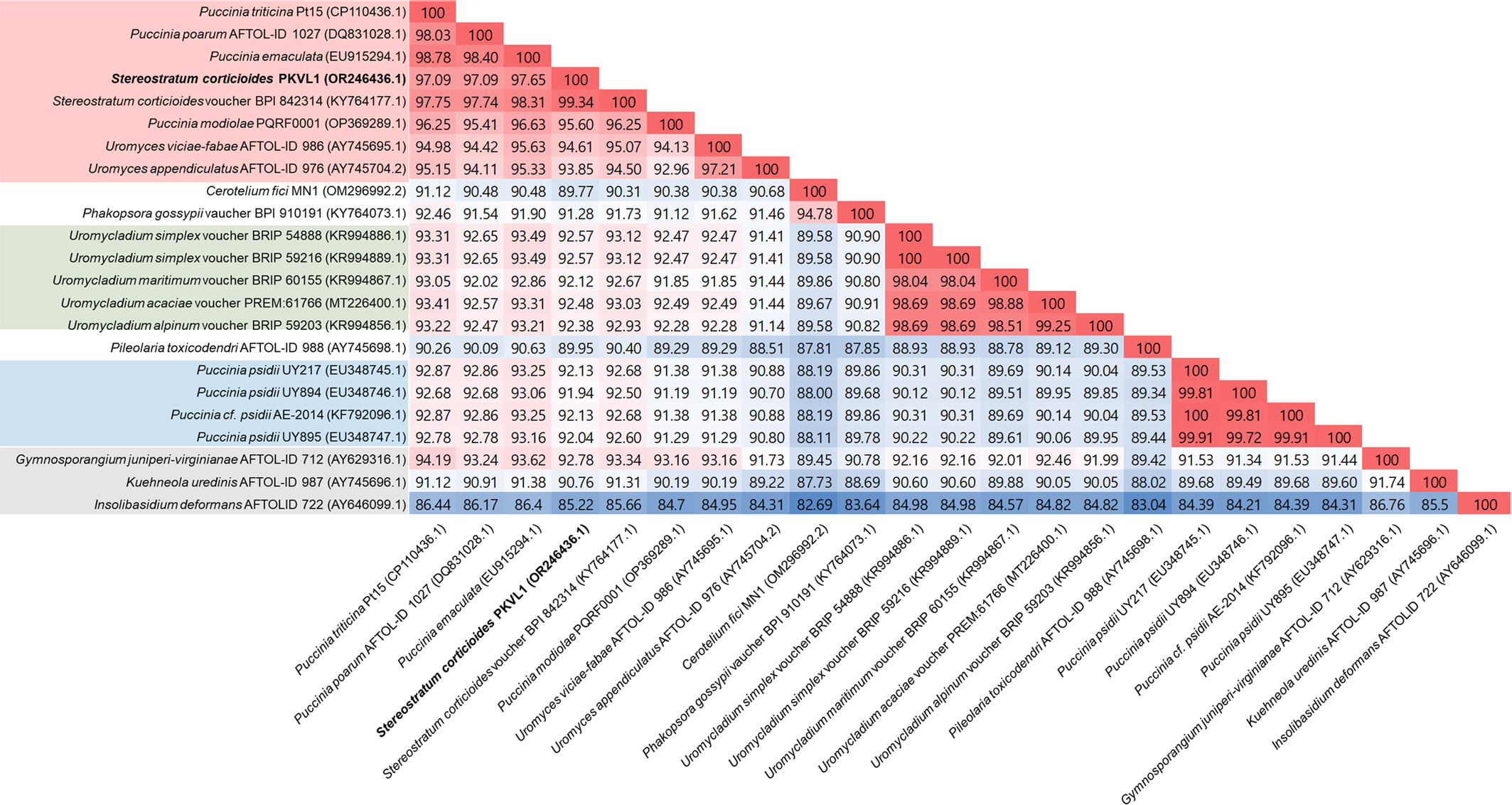
Okane, I., Ando, Y., Yamaoka, Y., Akiba, M. and Kubo, S. 2020. First report of heteroecism in
Stereostratum corticioides, the causal agent of bamboo culm rust.
Mycoscience 61: 172-178.

Spaulding, P. 1961. Foreign diseases of forest trees of the world. Agriculture handbooks no 197. US Department of Agriculture, Washington, DC, USA.
Tangjang, S., Reddy, M. S., Suryanarayanan, T. S. and Taka, T. 2018.
Stereostratum corticioides (Berk. & Broome) H. Magn. Rust on Phyllostachys bambusoides siebold & zucc. From Arunachal Pradesh, India. Curr. Sci. 115: 2011-2012.
The Korean Society of Plant Pathology. 2022. List of Plant Diseases in Korea. 6th ed. The Korean Society of Plant Pathology, Seoul, Korea.
Yu, N. H., Park, A. R., Yoon, H., Son, Y. K., Lee, B. H. and Kim, J. C. 2020. First report of rust disease on fringe tree by
Puccinia sp. and its alternative host.
Res. Plant Dis. 26: 179-182. (In Korean)






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print